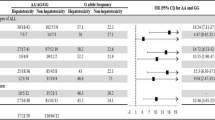
Key Points
-
Peripheral neurotoxicity is potentially permanent and ranks among the most common non-haematological adverse effects of chemotherapy
-
Identifying patients at high risk of chemotherapy-induced peripheral neurotoxicity (CIPN) is paramount
-
The identification of a clinical or genetic profile that can detect patients at high risk of CIPN still represents an unmet need
-
The methodology of pharmacogenetic studies on CIPN could be improved
-
Further studies are warranted to identify genetic associations that could inform the management of patients with CIPN
Abstract
The increasing availability of sophisticated methods to characterize human genetic variation has enabled pharmacogenetic data to be used not only to predict responses to treatment (in the context of so-called personalized medicine), but also to identify patients at high or low risk of specific treatment-related adverse effects. Over the past two decades, extensive attempts have been made to understand the genetic basis of chemotherapy-induced peripheral neurotoxicity (CIPN), one of the most severe non-haematological adverse effects of cancer treatment. Despite substantial efforts, however, the identification of a genetic profile that can detect patients at high risk of CIPN still represents an unmet need, as the information obtained from pharmacogenetic studies published so far is inconsistent at best. Among the reasons for these inconsistencies, methodological flaws and the poor reliability of existing tools for assessing CIPN features and severity are particularly relevant. This Review provides a critical update of the pharmacogenetics of CIPN, focusing on the studies published since 2011. Strategies for improving the reliability of future pharmacogenetic studies of CIPN are also discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Argyriou, A. A. et al. Clinical and electrophysiological features of peripheral neuropathy induced by administration of cisplatin plus paclitaxel-based chemotherapy. Eur. J. Cancer Care (Engl.) 16, 231–237 (2007).
Argyriou, A. A., Zolota, V., Kyriakopoulou, O. & Kalofonos, H. P. Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents. J. BUON 15, 435–446 (2010).
Alberti, P. & Cavaletti, G. Management of side effects in the personalized medicine era: chemotherapy-induced peripheral neuropathy. Methods Mol. Biol. 1175, 301–322 (2014).
Whirl-Carrillo, M. et al. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 92, 414–417 (2012).
Cavaletti, G., Alberti, P. & Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity in the era of pharmacogenomics. Lancet Oncol. 12, 1551–1561 (2011).
Argyriou, A. A., Kyritsis, A. P., Makatsoris, T. & Kalofonos, H. P. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag. Res. 6, 135–147 (2014).
Hershman, D. L. et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group clinical trials. J. Clin. Oncol. 34, 3014–3022 (2016).
Moriyama, B. et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 55, 290–297 (2012).
Argyriou, A. A. et al. Is advanced age associated with increased incidence and severity of chemotherapy-induced peripheral neuropathy? Support. Care Cancer 14, 223–229 (2006).
Kawakami, K. et al. Factors exacerbating peripheral neuropathy induced by paclitaxel plus carboplatin in non-small cell lung cancer. Oncol. Res. 20, 179–185 (2012).
Seretny, M. et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155, 2461–2470 (2014).
Beutler, A. S. et al. Sequencing of Charcot–Marie–Tooth disease genes in a toxic polyneuropathy. Ann. Neurol. 76, 727–737 (2014).
Boora, G. K. et al. Association of the Charcot–Marie–Tooth disease gene ARHGEF10 with paclitaxel induced peripheral neuropathy in NCCTG N08CA (Alliance). J. Neurol. Sci. 357, 35–40 (2015).
Argyriou, A. A. et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: results from a prospective multicenter study. Cancer 119, 3570–3577 (2013).
Lee, K. H. et al. Pharmacogenetic analysis of adjuvant FOLFOX for Korean patients with colon cancer. Cancer Chemother. Pharmacol. 71, 843–851 (2013).
Ruzzo, A. et al. Genetic markers for toxicity of adjuvant oxaliplatin and fluoropyrimidines in the phase III TOSCA trial in high-risk colon cancer patients. Sci. Rep. 4, 6828 (2014).
Custodio, A. et al. Pharmacogenetic predictors of severe peripheral neuropathy in colon cancer patients treated with oxaliplatin-based adjuvant chemotherapy: a GEMCAD group study. Ann. Oncol. 25, 398–403 (2014).
Kanai, M. et al. Large-scale prospective pharmacogenomics study of oxaliplatin-induced neuropathy in colon cancer patients enrolled in the JFMC41-1001-C2 (JOIN Trial). Ann. Oncol. 27, 1143–1148 (2016).
Sereno, M. et al. Genetic polymorphisms of SCN9A are associated with oxaliplatin-induced neuropathy. BMC Cancer 17, 63 (2017).
Won, H. H. et al. Polymorphic markers associated with severe oxaliplatin-induced, chronic peripheral neuropathy in colon cancer patients. Cancer 118, 2828–2836 (2012).
Terrazzino, S. et al. Genetic determinants of chronic oxaliplatin-induced peripheral neurotoxicity: a genome-wide study replication and meta-analysis. J. Peripher. Nerv. Syst. 20, 15–23 (2015).
Cecchin, E. et al. A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenomics J. 13, 403–409 (2013).
Kumamoto, K. et al. Polymorphisms of GSTP1. ERCC2 and TS-3′UTR are associated with the clinical outcome of mFOLFOX6 in colorectal cancer patients. Oncol. Lett. 6, 648–654 (2013).
Lamba, J. K. et al. Genetic variation in platinating agent and taxane pathway genes as predictors of outcome and toxicity in advanced non-small-cell lung cancer. Pharmacogenomics 15, 1565–1574 (2014).
Nishina, T. et al. A phase II clinical study of mFOLFOX6 plus bevacizumab as first-line therapy for Japanese advanced/recurrent colorectal cancer patients. Jpn. J. Clin. Oncol. 43, 1080–1086 (2013).
Fung, C. et al. Chemotherapy refractory testicular germ cell tumor is associated with a variant in armadillo repeat gene deleted in velco-cardio-facial syndrome (ARVCF). Front. Endocrinol. (Lausanne) 3, 163 (2012).
Joerger, M. et al. Germline polymorphisms in patients with advanced non-small cell lung cancer receiving first-line platinum-gemcitabine chemotherapy: a prospective clinical study. Cancer 118, 2466–2475 (2012).
Ale, A., Bruna, J., Navarro, X. & Udina, E. Neurotoxicity induced by antineoplastic proteasome inhibitors. Neurotoxicology 43, 28–35 (2014).
Argyriou, A. A., Cavaletti, G., Bruna, J., Kyritsis, A. P. & Kalofonos, H. P. Bortezomib-induced peripheral neurotoxicity: an update. Arch. Toxicol. 88, 1669–1679 (2014).
Dimopoulos, M. A., Sonneveld, P., Siegel, D., Palumbo, A. & San-Miguel, J. Carfilzomib and pomalidomide in patients with relapsed and/or refractory multiple myeloma with baseline risk factors. Ann. Oncol. 26, 2247–2256 (2015).
Argyriou, A. A., Bruna, J., Marmiroli, P. & Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit. Rev. Oncol. Hematol. 82, 51–77 (2012).
Roccaro, A. M., Vacca, A. & Ribatti, D. Bortezomib in the treatment of cancer. Recent Pat. Anticancer Drug Discov. 1, 397–403 (2006).
Coiffier, B. et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol. 12, 773–784 (2011).
Richardson, P. G. et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J. Clin. Oncol. 27, 3518–3525 (2009).
Favis, R. et al. Genetic variation associated with bortezomib-induced peripheral neuropathy. Pharmacogenet. Genomics 21, 121–129 (2011).
Corthals, S. L. et al. Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Haematologica 96, 1728–1732 (2011).
Van Ness, B. et al. Genomic variation in myeloma: design, content, and initial application of the Bank On A Cure SNP Panel to detect associations with progression-free survival. BMC Med. 6, 26 (2008).
Tacchetti, P. et al. Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: clinical and molecular analyses of a phase 3 study. Am. J. Hematol. 89, 1085–1091 (2014).
Page, S. H., Wright, E. K. Jr, Gama, L. & Clements, J. E. Regulation of CCL2 expression by an upstream TALE homeodomain protein-binding site that synergizes with the site created by the A-2578G SNP. PLoS ONE 6, e22052 (2011).
Magrangeas, F. et al. A genome-wide association study identifies a novel locus for bortezomib-induced peripheral neuropathy in European multiple myeloma patients. Clin. Cancer Res. 22, 4350–4355 (2016).
Campo, C. et al. Genetic susceptibility to bortezomib-induced peripheral neuroropathy: replication of the reported candidate susceptibility loci. Neurochem. Res. 42, 925–931 (2017).
Broyl, A. et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 11, 1057–1065 (2010).
García-Sanz, R. et al. Prediction of peripheral neuropathy in multiple myeloma patients receiving bortezomib and thalidomide: a genetic study based on a single nucleotide polymorphism array. Hematol. Oncol. http://dx.doi.org/10.1002/hon.2337 (2016).
Zhou, W., An, G., Jian, Y., Guo, H. & Chen, W. Effect of CYP2C19 and CYP3A4 gene polymorphisms on the efficacy of bortezomib-based regimens in patients with multiple myeloma. Oncol. Lett. 10, 1171–1175 (2015).
Abraham, J. E. et al. Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel. Clin. Cancer Res. 20, 2466–2475 (2014).
Boora, G. K. et al. Testing of candidate single nucleotide variants associated with paclitaxel neuropathy in the trial NCCTG N08C1 (Alliance). Cancer Med. 5, 631–639 (2016).
Baldwin, R. M. et al. A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin. Cancer Res. 18, 5099–5109 (2012).
Leandro-García, L. J. et al. Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J. Med. Genet. 50, 599–605 (2013).
Schneider, B. P. et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin. Cancer Res. 21, 5082–5091 (2015).
Wheeler, H. E. et al. Integration of cell line and clinical trial genome-wide analyses supports a polygenic architecture of paclitaxel-induced sensory peripheral neuropathy. Clin. Cancer Res. 19, 491–499 (2013).
Hertz, D. L. et al. Pharmacogenetic discovery in CALGB (Alliance) 90401 and mechanistic validation of a VAC14 polymorphism that increases risk of docetaxel-induced neuropathy. Clin. Cancer Res. 22, 4890–4900 (2016).
Eckhoff, L. et al. Docetaxel-induced neuropathy: a pharmacogenetic case–control study of 150 women with early-stage breast cancer. Acta Oncol. 54, 530–537 (2015).
Park, S. B. et al. Paclitaxel-induced neuropathy: potential association of MAPT and GSK3B genotypes. BMC Cancer 14, 993 (2014).
Lee, S. Y. et al. Genetic polymorphisms of SLC28A3, SLC29A1 and RRM1 predict clinical outcome in patients with metastatic breast cancer receiving gemcitabine plus paclitaxel chemotherapy. Eur. J. Cancer 50, 698–705 (2014).
Jordan, M. A. & Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265 (2004).
Lobert, S., Vulevic, B. & Correia, J. J. Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine. Biochemistry 35, 6806–6814 (1996).
Egbelakin, A. et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 56, 361–367 (2011).
Moore, A. S. et al. Vincristine pharmacodynamics and pharmacogenetics in children with cancer: a limited-sampling, population modelling approach. J. Paediatr. Child Health 47, 875–882 (2011).
Sims, R. P. The effect of race on the CYP3A-mediated metabolism of vincristine in pediatric patients with acute lymphoblastic leukemia. J. Oncol. Pharm. Pract. 22, 76–81 (2016).
Guilhaumou, R. et al. Impact of plasma and intracellular exposure and CYP3A4. CYP3A5, and ABCB1 genetic polymorphisms on vincristine-induced neurotoxicity. Cancer Chemother. Pharmacol. 68, 1633–1638 (2011).
Ceppi, F. et al. Polymorphisms of the vincristine pathway and response to treatment in children with childhood acute lymphoblastic leukemia. Pharmacogenomics 15, 1105–1116 (2014).
Lopez-Lopez, E. et al. Vincristine pharmacokinetics pathway and neurotoxicity during early phases of treatment in pediatric acute lymphoblastic leukemia. Pharmacogenomics 17, 731–741 (2016).
Diouf, B. et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 313, 815–823 (2015).
Stock, W. et al. An inherited genetic variant in CEP72 promoter predisposes to vincristine-induced peripheral neuropathy in adults with acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 101, 391–395 (2017).
Gutierrez-Camino, A. et al. Lack of association of the CEP72 rs924607 TT genotype with vincristine-related peripheral neuropathy during the early phase of pediatric acute lymphoblastic leukemia treatment in a Spanish population. Pharmacogenet. Genomics 26, 100–102 (2016).
Johnson, D. C. et al. Genetic factors underlying the risk of thalidomide-related neuropathy in patients with multiple myeloma. J. Clin. Oncol. 29, 797–804 (2011).
Cibeira, M. T. et al. Impact on response and survival of DNA repair single nucleotide polymorphisms in relapsed or refractory multiple myeloma patients treated with thalidomide. Leuk. Res. 35, 1178–1183 (2011).
Relling, M. V. & Evans, W. E. Pharmacogenomics in the clinic. Nature 526, 343–350 (2015).
Relling, M. V. et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin. Pharmacol. Ther. 93, 324–325 (2013).
Relling, M. V. et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin. Pharmacol. Ther. 89, 387–391 (2011).
Ross, S., Anand, S. S., Joseph, P. & Paré, G. Promises and challenges of pharmacogenetics: an overview of study design, methodological and statistical issues. JRSM Cardiovasc. Dis. http://dx.doi.org/10.1258/cvd.2012.012001 (2012).
Acknowledgements
The authors' research work was supported by grant 2013–0842 from Fondazione Cariplo (to A.A.G. and G.C.), by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC) Progetto IG 2016 Id.18631 (to G.C.) and partially by grant PI1501303 from Instituto de Salud Carlos III (ISCIII) and Fondo Europeo de Desarrollo Regional (FEDER) to J.B.
Author information
Authors and Affiliations
Contributions
Each author contributed to all aspects of the preparation of this Review (researching data for the article, writing the manuscript, discussions of content and review or editing of the article before submission).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Pharmacogenetic studies related to chemotherapy-induced peripheral neurotoxicity (CIPN) in patients treated with platinum compounds. (DOC 52 kb)
Supplementary information S2 (table)
Pharmacogenetic studies related to chemotherapy-induced peripheral neurotoxicity (CIPN) in patients treated with bortezomib. (DOC 50 kb)
Supplementary information S3 (table)
Pharmacogenetic studies related to chemotherapy-induced peripheral neurotoxicity (CIPN) in patients treated with taxanes. (DOC 51 kb)
Supplementary information S4 (table)
Pharmacogenetic studies related to chemotherapy-induced peripheral neurotoxicity (CIPN) in patients treated with thalidomide or vincristine. (DOC 49 kb)
Rights and permissions
About this article
Cite this article
Argyriou, A., Bruna, J., Genazzani, A. et al. Chemotherapy-induced peripheral neurotoxicity: management informed by pharmacogenetics. Nat Rev Neurol 13, 492–504 (2017). https://doi.org/10.1038/nrneurol.2017.88
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.88
This article is cited by
-
Whole genome sequencing across clinical trials identifies rare coding variants in GPR68 associated with chemotherapy-induced peripheral neuropathy
Genome Medicine (2023)
-
Bortezomib induced peripheral neuropathy and single nucleotide polymorphisms in PKNOX1
Biomarker Research (2023)
-
Chemotherapy and peripheral neuropathy
Neurological Sciences (2021)
-
The future of myeloma precision medicine: integrating the compendium of known drug resistance mechanisms with emerging tumor profiling technologies
Leukemia (2019)
-
Is a pharmacogenomic panel useful to estimate the risk of oxaliplatin-related neurotoxicity in colorectal cancer patients?
The Pharmacogenomics Journal (2019)