Abstract
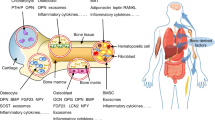
Emerging evidence points to a critical role for the skeleton in several homeostatic processes, including energy balance. The connection between fuel utilization and skeletal remodeling begins in the bone marrow with lineage allocation of mesenchymal stem cells to adipocytes or osteoblasts. Mature bone cells secrete factors that influence insulin sensitivity, and fat cells synthesize cytokines that regulate osteoblast differentiation; thus, these two pathways are closely linked. The emerging importance of the bone–fat interaction suggests that novel molecules could be used as targets to enhance bone formation and possibly prevent fractures. In this article, we discuss three pathways that could be pharmacologically targeted for the ultimate goal of enhancing bone mass and reducing osteoporotic fracture risk: the leptin, peroxisome proliferator-activated receptor gamma and osteocalcin pathways. Not surprisingly, because of the complex interactions across homeostatic networks, other pathways will probably be activated by this targeting, which could prove to be beneficial or detrimental for the organism. Hence, a more complete picture of energy utilization and skeletal remodeling will be required to bring any potential agents into the future clinical armamentarium.
Key Points
-
Bone and fat arise from the same mesenchymal stem cells in the bone marrow
-
Osteoblasts secrete factors that regulate insulin production and adipocyte sensitivity to insulin
-
Leptin is an adipokine that acts through the hypothalamus to regulate appetite and bone remodeling
-
Peripheral fat stores are hormonally active and might regulate bone turnover
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Flier, J. S. Clinical review 94: What's in a name? In search of leptin's physiologic role. J. Clin. Endocrinol. Metab. 83, 1407–1413 (1998).
Serre, C. M., Farlay, D., Delmas, P. D. & Chenu, C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25, 623–629 (1999).
Takeda, S. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002).
Patel, M. S. & Elefteriou, F. The new field of neuroskeletal biology. Calcif. Tissue Int. 80, 337–347 (2007).
Ducy, P. et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000).
Elefteriou, F. et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434, 514–520 (2005).
Elefteriou, F. et al. Serum leptin level is a regulator of bone mass. Proc. Natl Acad. Sci. USA 101, 3258–3263 (2004).
Thomas, T. et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140, 1630–1638 (1999).
Steppan, C. M., Crawford, D. T., Chidsey Frink, K. L., Ke, H. & Swick, A. G. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul. Pept. 92, 73–78 (2000).
Cornish, J. et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J. Endocrinol. 175, 405–415 (2002).
Hamrick, M. W., Pennington, C., Newton, D., Xie, D. & Isales, C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34, 376–383 (2004).
Kim, S. M. et al. Association of leptin receptor polymorphisms Lys109Arg and Gln223Arg with serum leptin profile and bone mineral density in Korean women. Am. J. Obstet. Gynecol. 198, 421 e1–e8 (2008).
Koh, J. M. et al. Estrogen receptor α gene polymorphisms (Pvu II and Xba I) influence association between leptin receptor gene polymorphism (Gln223Arg) and bone mineral density in young men. Eur. J. Endocrinol. 147, 777–783 (2002).
Richert, L. et al. Bone mass in prepubertal boys is associated with a Gln223Arg amino acid substitution in the leptin receptor. J. Clin. Endocrinol. Metab. 92, 4380–4386 (2007).
Crabbe, P. et al. Are serum leptin and the Gln223Arg polymorphism of the leptin receptor determinants of bone homeostasis in elderly men? Eur. J. Endocrinol. 154, 707–714 (2006).
Fairbrother, U. L. et al. Leptin receptor genotype at Gln223Arg is associated with body composition, BMD, and vertebral fracture in postmenopausal Danish women. J. Bone Miner. Res. 22, 544–550 (2007).
Weiss, L. A., Barrett-Connor, E., von Muhlen, D. & Clark, P. Leptin predicts BMD and bone resorption in older women but not older men: the Rancho Bernardo study. J. Bone Miner. Res. 21, 758–764 (2006).
Thomas, T. et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone 29, 114–120 (2001).
Yamauchi, M. et al. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin. Endocrinol. (Oxf.) 55, 341–347 (2001).
Blain, H. et al. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J. Clin. Endocrinol. Metab. 87, 1030–1035 (2002).
Sato, M. et al. Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J. Clin. Endocrinol. Metab. 86, 5273–5276 (2001).
Lorentzon, M., Landin, K., Mellstrom, D. & Ohlsson, C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J. Bone Miner. Res. 21, 1871–1878 (2006).
Filip, R. & Raszewski, G. Bone mineral density and bone turnover in relation to serum leptin, alpha-ketoglutarate and sex steroids in overweight and obese postmenopausal women. Clin. Endocrinol. (Oxf.) 70, 214–220 (2009).
Jürimäe, J. & Jürimäe, T. Influence of insulin-like growth factor-1 and leptin on bone mineral content in healthy premenopausal women. Exp. Biol. Med. (Maywood) 231, 1673–1677 (2006).
Jürimäe, J., Jürimäe, T., Leppik, A. & Kums, T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J. Bone Miner. Metab. 26, 618–623 (2008).
Bonnet, N., Pierroz, D. D. & Ferrari, S. L. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J. Musculoskelet. Neuronal Interact. 8, 94–104 (2008).
Takeda, S. & Karsenty, G. Molecular bases of the sympathetic regulation of bone mass. Bone 42, 837–840 (2008).
Elefteriou, F. Neuronal signaling and the regulation of bone remodeling. Cell. Mol. Life Sci. 62, 2339–2349 (2005).
Gat-Yablonski, G. et al. Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology 145, 343–350 (2004).
Kishida, Y. et al. Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone 37, 607–621 (2005).
Lorentzon, R., Alehagen, U. & Boquist, L. Osteopenia in mice with genetic diabetes. Diabetes Res. Clin. Pract. 2, 157–163 (1986).
Ealey, K. N., Fonseca, D., Archer, M. C. & Ward, W. E. Bone abnormalities in adolescent leptin-deficient mice. Regul. Pept. 136, 9–13 (2006).
Hamrick, M. W., Ding, K. H., Ponnala, S., Ferrari, S. L. & Isales, C. M. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J. Bone Miner. Res. 23, 870–878 (2008).
Engelbregt, M. J. et al. Body composition and bone measurements in intra-uterine growth retarded and early postnatally undernourished male and female rats at the age of 6 months: comparison with puberty. Bone 34, 180–186 (2004).
Friedman, S. M. et al. Growth deceleration and bone metabolism in nutritional dwarfing rats. Int. J. Food Sci. Nutr. 52, 225–233 (2001).
Tatsumi, S., Ito, M., Asaba, Y., Tsutsumi, K. & Ikeda, K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology 149, 634–641 (2008).
Boyer, P. M. et al. Bone status in an animal model of chronic sub-optimal nutrition: a morphometric, densitometric and mechanical study. Br. J. Nutr. 93, 663–669 (2005).
Handler, P., Baylin, G. J. & Follis, R. H. Jr. The effects of caloric restriction on skeletal growth: four figures. J. Nutr. 34, 677–689 (1947).
LaMothe, J. M., Hepple, R. T. & Zernicke, R. F. Selected contribution: bone adaptation with aging and long-term caloric restriction in Fischer 344 × Brown-Norway F1-hybrid rats. J. Appl. Physiol. 95, 1739–1745 (2003).
Lambert, J., Lamothe, J. M., Zernicke, R. F., Auer, R. N. & Reimer, R. A. Dietary restriction does not adversely affect bone geometry and mechanics in rapidly growing male wistar rats. Pediatr. Res. 57, 227–231 (2005).
Berrigan, D. et al. Phenotypic effects of calorie restriction and insulin-like growth factor-1 treatment on body composition and bone mineral density of C57BL/6 mice: implications for cancer prevention. In Vivo 19, 667–674 (2005).
Ferguson, V. L., Greenberg, A. R., Bateman, T. A., Ayers, R. A. & Simske, S. J. The effects of age and dietary restriction without nutritional supplementation on whole bone structural properties in C57BL/6J mice. Biomed. Sci. Instrum. 35, 85–91 (1999).
Bouxsein, M. L. et al. Mice lacking beta-adrenergic receptors have increased bone mass, but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology 150, 144–152 (2009).
Shi, Y. et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc. Natl Acad. Sci. USA 105, 20529–20533 (2008).
Holloway, W. R. et al. Leptin inhibits osteoclast generation. J. Bone Miner. Res. 17, 200–209 (2002).
Gimble, J. M., Zvonic, S., Floyd, Z. E., Kassem, M. & Nuttall, M. E. Playing with bone and fat. J. Cell. Biochem. 98, 251–266 (2006).
Hamrick, M. W. & Ferrari, S. L. Leptin and the sympathetic connection of fat to bone. Osteoporos. Int. 19, 905–912 (2008).
Martin, A. et al. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology 146, 3652–3659 (2005).
Baek, K. & Bloomfield, S. A. Beta-adrenergic blockade and leptin replacement effectively mitigate disuse bone loss. J. Bone Miner. Res. doi:10.1359/jbmr.081241.
Hamrick, M. W. Leptin, bone mass, and the thrifty phenotype. J. Bone Miner. Res. 19, 1607–1611 (2004).
Martin, A. et al. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology 148, 3419–3425 (2007).
Welt, C. K. et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl J. Med. 351, 987–997 (2004).
Meisinger, C., Heier, M., Lang, O. & Doring, A. Beta-blocker use and risk of fractures in men and women from the general population: the MONICA/KORA Augsburg cohort study. Osteoporos. Int. 18, 1189–1195 (2007).
Pasco, J. A. et al. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J. Bone Miner. Res. 19, 19–24 (2004).
Bonnet, N. et al. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40, 1209–1216 (2007).
Reid, I. R. et al. Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J. Clin. Endocrinol. Metab. 90, 5212–5216 (2005).
Kliewer, S. A. et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl Acad. Sci. USA 91, 7355–7359 (1994).
Knouff, C. & Auwerx, J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr. Rev. 25, 899–918 (2004).
Cock, T. A., Houten, S. M. & Auwerx, J. Peroxisome proliferator-activated receptor-gamma: too much of a good thing causes harm. EMBO Rep. 5, 142–147 (2004).
Hagman, J., Belanger, C., Travis, A., Turck, C. W. & Grosschedl, R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 7, 760–773 (1993).
Jimenez, M. A., Akerblad, P., Sigvardsson, M. & Rosen, E. D. Critical role for Ebf1and Ebf2 in the adipogenic transcriptional cascade. Mol. Cell. Biol. 27, 743–757 (2007).
Elbrecht, A. et al. Molecular cloning, expression and characterization of human peroxisome proliferators activated receptors gamma 1 and gamma 2. Biochem. Biophys. Res. Commun. 224, 431–437 (1996).
Gimble, J. M. et al. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol. Pharmacol. 50, 1087–1094 (1996).
Lecka-Czernik, B. et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J. Cell. Biochem. 74, 357–371 (1999).
Shockley, K. R. et al. PPARγ2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J. Cell. Biochem. 106, 232–246 (2009).
Akune, T. et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 113, 846–855 (2004).
Wan, Y., Chong, L. W. & Evans, R. M. PPAR-γ regulates osteoclastogenesis in mice. Nat. Med. 13, 1496–1503 (2007).
Cock, T. A. et al. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep. 5, 1007–1012 (2004).
Tornvig, L., Mosekilde, L., Justesen, J., Falk, E. & Kassem, M. Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif. Tissue Int. 69, 46–50 (2001).
Aleo, M. D. et al. Mechanism and implications of brown adipose tissue proliferation in rats and monkeys treated with the thiazolidinedione darglitazone, a potent peroxisome proliferator-activated receptor-γ agonist. J. Pharmacol. Exp. Ther. 305, 1173–1182 (2003).
Li, M. et al. Surface-specific effects of a PPAR agonist, darglitazone, on bone in mice. Bone 39, 796–806 (2006).
Lazarenko, O., Rzonca, S., Suva, L. & Lecka-Czernik, B. Netoglitazone is a PPAR gamma ligand with selective effects on bone and fat. Bone 38, 74–84 (2006).
Ackert-Bicknell, C. L. et al. Strain specific effects of Rosiglitazone on bone mass, body composition and serum insulin-like growth factor-I. Endocrinology 150, 1330–1340 (2008).
Gerstein, H. et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368, 1096–1105 (2006).
Yki-Järvinen, H. Thiazolidinediones. N. Engl. J. Med. 351, 1106–1118 (2004).
Krentz, A. & Bailey, C. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 65, 385–411 (2005).
Schwartz, A. V. et al. Thiazolidinedione (TZD) use and bone loss in older diabetic adults. J. Clin. Endocrinol. Metab. 91, 3349–3354 (2006).
Grey, A. et al. The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 92, 1305–1310 (2007).
Kahn, S. E. et al. Rosiglitazone-associated fractures in type 2 diabetes: an analysis from ADOPT. Diabetes Care 31, 845–851 (2008).
Meier, C. et al. Use of thiazolidinediones and fracture risk. Arch. Intern. Med. 168, 820–825 (2008).
Schwartz, A. V. TZDs and bone: a review of the recent clinical evidence. PPAR Res. 2008, 297893 (2008).
Rosen, C. J. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab. 7, 7–10 (2008).
Bonds, D. E. et al. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 91, 3404–3410 (2006).
Melton, L. J. 3rd et al. A bone structural basis for fracture risk in diabetes. J. Clin. Endocrinol. Metab. 93, 4804–4809 (2008).
Lawson, E. A. & Klibanski, A. Endocrine abnormalities in anorexia nervosa. Nat. Clin. Pract. Endocrinol. Metab. 4, 407–414 (2008).
Rosen, C. J. & Klibanski, A. Bone, fat and body composition: evolving concepts in the pathogenesis of osteoporosis. Am. J. Med. 122, 409–414 (2009).
Cui, Q., Wang, G. J. & Balian, G. Pluripotential marrow cells produce adipocytes when transplanted into steroid-treated mice. Connect. Tissue. Res. 41, 45–56 (2000).
van Staa, T. P., Leufkens, H. G. & Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos. Int. 13, 777–787 (2002).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007).
Hauschka, P. V., Lian, J. B., Cole, D. E. & Gundberg, C. M. Osteocalcin and matriz Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev. 69, 990–1047 (1989).
Murshed, M., Schinke, T., McKee, M. D. & Karsenty, G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell. Biol. 165, 625–630 (2004).
Ferron, M., Hinoi, E., Karsenty, G. & Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl Acad. Sci. USA 105, 5266–5270 (2008).
Hinoi, E. et al. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J. Cell. Biol. 183, 1235–1242 (2008).
Covey, S. D. et al. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell. Metab. 4, 291–302 (2006).
Morioka, T. et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J. Clin. Invest. 117, 2753–2756 (2007).
Pittas, A. G., Harris, S. S., Eliades, M., Stark, P. & Dawson-Hughes, B. Association between serum osteocalcin and markers of metabolic phenotype. J. Clin. Endocrinol. Metab. 94, 827–832 (2009).
Kanazawa, I. et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 94, 45–49 (2009).
Kindblom, J. M. et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J. Bone Miner. Res. doi:10.1359/jbmr.081234.
Delmas, P. D. Biochemical markers of bone turnover for the clinical investigation of osteoporosis. Osteoporos. Int. 3 (Suppl. 1), 81–86 (1993).
Boskey, A. L. et al. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 23, 187–196 (1998).
Yadav, V. K. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837 (2008).
Rosen, C. J. Serotonin rising—the bone, brain, bowel connection. N. Engl. J. Med. 360, 957–959 (2009).
Rosen, C. J., Ackert-Bicknell, C. & Rodriguez, J. P. Marrow fat and the bone micro-environment: developmental, functional, and pathological implications. Crit. Rev. Eukaryot. Gene Expr. (in press).
Olmsted-Davis, E. et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am. J. Pathol. 170, 620–632 (2007).
Acknowledgements
This work was supported by NIH NIAMS grants AR 45433, 54604 to C. J. Rosen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kawai, M., Devlin, M. & Rosen, C. Fat targets for skeletal health. Nat Rev Rheumatol 5, 365–372 (2009). https://doi.org/10.1038/nrrheum.2009.102
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.102
This article is cited by
-
Comparison of clinical outcome of lumbar spinal stenosis surgery in patients with and without osteoporosis: a prospective cohort study
Journal of Orthopaedic Surgery and Research (2023)
-
Deletion of Mettl3 in mesenchymal stem cells promotes acute myeloid leukemia resistance to chemotherapy
Cell Death & Disease (2023)
-
Bone mineral density and normal-weight obesity syndrome: beyond body weight and body mass index
Journal of Bone and Mineral Metabolism (2023)
-
The m6A demethylase FTO promotes the osteogenesis of mesenchymal stem cells by downregulating PPARG
Acta Pharmacologica Sinica (2022)
-
Adiposity and bone microarchitecture in the GLOW study
Osteoporosis International (2021)