Abstract
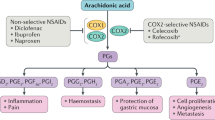
Cyclo-oxygenase (COX) inhibitors are widely used to relieve musculoskeletal pain. These agents block the production of prostaglandins (PGs) at sites of inflammation by inhibiting the activity of two COX enzymes necessary for PG production and normal organ homeostasis. Inhibition of PG production at sites unrelated to pain is associated with adverse drug reactions (ADRs). The degree of analgesic efficacy, as well as the incidence and the localization of ADRs, are critically influenced by the pharmacokinetics (absorption, distribution and elimination) of these drugs. Ideally, sufficient and permanent inhibition of COX enzymes should be achieved in target tissues, with minimal ADRs. To minimize underdosing or overdosing, which result in therapeutic failure or ADRs, the COX inhibitor with the most appropriate pharmacokinetic properties should be selected on the basis of a thorough pharmacokinetic–pharmacodynamic analysis. In this Review, the pharmacokinetics of the prevailing COX inhibitors will be discussed and enigmatic aspects of these intensively used drugs will be considered.
Key Points
-
Cyclo-oxygenase (COX) inhibitors comprise substances with a broad spectrum of pharmacokinetic characteristics
-
Rapid onset of analgesia requires administration of COX inhibitors with fast absorption, such as solutions or uncoated tablets with salts of the respective compound
-
Among the COX inhibitors, only acidic compounds are currently considered to accumulate in the inflamed joint (deep compartment) and to confer long-term inhibition of COX2 when given at therapeutic doses
-
To achieve acute analgesic effects and to avoid toxic drug accumulation following long-term use, administration of a suitable initial loading dose followed by a smaller maintenance dose should be considered
-
Only >95% COX1 inhibition in platelets confers a clinically relevant inhibition of platelet aggregation; moreover, correlation analyses suggest that 80% COX2 inhibition is necessary for pain relief
-
Overdosing increases the incidence of adverse effects, but does not enhance analgesia
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hughes, D. A. & Aronson, J. K. A systematic review and empirical analysis of the relation between dose and duration of drug action. J. Clin. Pharmacol. 50, 17–26 (2010).
Greenblatt, D. J. Elimination half-life of drugs: value and limitations. Annu. Rev. Med. 36, 421–427 (1985).
Levy, G. & Gibaldi, M. Pharmacokinetics of drug action. Annu. Rev. Pharmacol. 12, 85–98 (1972).
Fu, J. Y., Masferrer, J. L., Seibert, K., Raz, A. & Needleman, P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 265, 16737–16740 (1990).
Xie, W. L., Chipman, J. G., Robertson, D. L., Erikson, R. L. & Simmons, D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl Acad. Sci. USA 88, 2692–2696 (1991).
Ahmadi, S., Lippross, S., Neuhuber, W. L. & Zeilhofer, H. U. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat. Neurosci. 5, 34–40 (2002).
Zeilhofer, H. U. Prostanoids in nociception and pain. Biochem. Pharmacol. 73, 165–174 (2007).
Grosser, T., Fries, S. & FitzGerald, G. A. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 116, 4–15 (2006).
Patrono, C. & Baigent, C. Low-dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Mol. Interv. 9, 31–39 (2009).
Hinz, B. & Brune, K. Can drug removals involving cyclooxygenase-2 inhibitors be avoided? A plea for human pharmacology. Trends Pharmacol. Sci. 29, 391–397 (2008).
Aronoff, D. M., Boutaud, O., Marnett, L. J. & Oates, J. A. Inhibition of prostaglandin H2 synthases by salicylate is dependent on the oxidative state of the enzymes. J. Pharmacol. Exp. Ther. 304, 589–595 (2003).
Palmer, J. D., Sparrow, O. C. & Iannotti, F. Postoperative hematoma: a 5-year survey and identification of avoidable risk factors. Neurosurgery 35, 1061–1064 (1994).
Huntjens, D. R., Danhof, M. & Della Pasqua, O. E. Pharmacokinetic-pharmacodynamic correlations and biomarkers in the development of COX-2 inhibitors. Rheumatology (Oxford) 44, 846–859 (2005).
Capone, M. L. et al. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J. Am. Coll. Cardiol. 45, 1295–1301 (2005).
Catella-Lawson, F. et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 345, 1809–1817 (2001).
MacDonald, T. M. & Wei, L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet 361, 573–574 (2003).
US Department of Health and Human Services Information for Healthcare Professionals: Concomitant Use of Ibuprofen and Aspirin, [online] (2006).
Wilner, K. D. et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J. Clin. Pharmacol. 42, 1027–1030 (2002).
Gladding, P. A. et al. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am. J. Cardiol. 101, 1060–1063 (2008).
Greenberg, H. E. et al. A new cyclooxygenase-2 inhibitor, rofecoxib (VIOXX), did not alter the antiplatelet effects of low-dose aspirin in healthy volunteers. J. Clin. Pharmacol. 40, 1509–1515 (2000).
Dallob, A. et al. Characterization of etoricoxib, a novel, selective COX-2 inhibitor. J. Clin. Pharmacol. 43, 573–585 (2003).
Ouellet, M., Riendeau, D. & Percival, M. D. A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin. Proc. Natl Acad. Sci. USA 98, 14583–14588 (2001).
Rimon, G. et al. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. Proc. Natl Acad. Sci. USA 107, 28–33 (2010).
Brune, K. & Hinz, B. Selective cyclooxygenase-2 inhibitors: similarities and differences. Scand. J. Rheumatol. 33, 1–6 (2004).
Hinz, B., Renner, B. & Brune, K. Drug insight: cyclo-oxygenase-2 inhibitors—a critical appraisal. Nat. Clin. Pract. Rheumatol. 3, 552–560 (2007).
Geisslinger, G., Menzel, S., Wissel, K. & Brune, K. Single dose pharmacokinetics of different formulations of ibuprofen and aspirin. Drug Invest. 5, 238–242 (1993).
Fulkerson, J. P. & Folcik, M. A. Analgesia following arthroscopic surgery: comparison of diflunisal and acetaminophen with codeine. Arthroscopy 2, 108–110 (1986).
Werner, U. et al. Celecoxib inhibits metabolism of cytochrome P450 2D6 substrate metoprolol in humans. Clin. Pharmacol. Ther. 74, 130–137 (2003).
Laska, E. M. et al. The correlation between blood levels of ibuprofen and clinical analgesic response. Clin. Pharmacol. Ther. 40, 1–7 (1986).
Farkouh, M. E. et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 364, 675–684 (2004).
Stüber, W. et al. Untersuchungen zur Bioverfügbarkeit von Diclofenac-Natrium aus einer neu entwickelten magensaftresistenten Pellet-Zubereitung. Pharm. Ztg 133, 29–35 (1988).
Colombo, G., Giombini, A., Pamich, T., Peruzzi, E. & Pisati, R. Diclofenac dispersible provides superior analgesia with faster onset of action compared to naproxen granular in patients with acute, painful, minor sports injuries. J. Sports Med. Phys. Fitness 37, 228–233 (1997).
Griffin, M. R. et al. High frequency of use of rofecoxib at greater than recommended doses: cause for concern. Pharmacoepidemiol. Drug Saf. 13, 339–343 (2004).
Roumie, C. L., Arbogast, P. G., Mitchel, E. F. Jr & Griffin, M. R. Prescriptions for chronic high-dose cyclooxygenase-2 inhibitors are often inappropriate and potentially dangerous. J. Gen. Intern. Med. 20, 879–883 (2005).
Renner, B. et al. Absorption and distribution of etoricoxib in plasma, CSF and wound tissue in patients following hip surgery—a pilot study. Naunyn–Schmiedeberg's Arch. Pharmacol. 381, 127–136 (2010).
Malmstrom, K. et al. Etoricoxib in acute pain associated with dental surgery: a randomized, double-blind, placebo- and active comparator-controlled dose-ranging study. Clin. Ther. 26, 667–679 (2004).
Simmons, D. L., Botting, R. M. & Hla, T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56, 387–437 (2004).
Brune, K. Persistence of NSAIDs at effect sites and rapid disappearance from side-effect compartments contributes to tolerability. Curr. Med. Res. Opin. 23, 2985–2995 (2007).
Brune, K. & Furst, D. E. Combining enzyme specificity and tissue selectivity of cyclooxygenase inhibitors: towards better tolerability? Rheumatology (Oxford) 46, 911–919 (2007).
Day, R. O., McLachlan, A. J., Graham, G. G. & Williams, K. M. Pharmacokinetics of nonsteroidal anti-inflammatory drugs in synovial fluid. Clin. Pharmacokinet. 36, 191–210 (1999).
Graf, P., Glatt, M. & Brune, K. Acidic nonsteroid anti-inflammatory drugs accumulating in inflamed tissue. Experientia 31, 951–953 (1975).
Dutta, S. K., Basu, S. K. & Sen, K. K. Binding of diclofenac sodium with bovine serum albumin at different tuemperatures, pH and ionic strengths. Indian J. Exp. Biol. 44, 123–127 (2006).
Fowler, P. D., Shadforth, M. F., Crook, P. R. & John, V. A. Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis. Eur. J. Clin. Pharmacol. 25, 389–394 (1983).
Doyle, M. J., Eichhold, T. H., Loomans, M. E., Farmer, R. W. & Kelm, G. R. Comparison of tebufelone distribution in rat blood plasma and inflamed and noninflamed tissues following peroral and intravenous administration. J. Pharm. Sci. 82, 847–850 (1993).
Schweitzer, A., Hasler-Nguyen, N. & Zijlstra, J. Preferential uptake of the non steroid anti-inflammatory drug diclofenac into inflamed tissues after a single oral dose in rats. BMC Pharmacol. 9, 5 (2009).
Elmquist, W. F., Chan, K. K. & Sawchuk, R. J. Transsynovial drug distribution: synovial mean transit time of diclofenac and other nonsteroidal antiinflammatory drugs. Pharm. Res. 11, 1689–1697 (1994).
Scott, G. et al. Pharmacokinetics of lumiracoxib in plasma and synovial fluid. Clin. Pharmacokinet. 43, 467–478 (2004).
Schrör, K., Mehta, P. & Mehta, J. L. Cardiovascular risk of selective cyclooxygenase-2 inhibitors. J. Cardiovasc. Pharmacol. Ther. 10, 95–101 (2005).
McAdam, B. F. et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl Acad. Sci. USA 96, 272–277 (1999).
Brune, K., Rainsford, K. D. & Schweitzer, A. Biodistribution of mild analgesics. Br. J. Clin. Pharmacol. 110 (Suppl. 2), 279S–284S (1980).
Bianchi, M., Broggini, M., Balzarini, P., Franchi, S. & Sacerdote, P. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int. J. Clin. Pract. 61, 1270–1277 (2007).
Maier, T. J. et al. Cellular membranes function as a storage compartment for celecoxib. J. Mol. Med. 87, 981–993 (2009).
Brune, K. & Alpermann, H. Non-acidic pyrazoles: inhibition of prostaglandin production, carrageenan edema and yeast fever. Agents Actions 13, 360–363 (1983).
Tatsuo, M. A. et al. Analgesic and antiinflammatory effects of dipyrone in rat adjuvant arthritis model. Inflammation 18, 399–405 (1994).
Hinz, B., Cheremina, O. & Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 22, 383–390 (2008).
Hinz, B. et al. Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans: new insights into the pharmacology of an old analgesic. FASEB J. 21, 2343–2351 (2007).
Boardman, P. L. & Hart, F. D. Clinical measurement of the anti-inflammatory effects of salicylates in rheumatoid arthritis. BMJ 4, 264–268 (1967).
Ring, E. F., Collins, A. J., Bacon, P. A. & Cosh, J. A. Quantitation of thermography in arthritis using multi-isothermal analysis. II. Effect of nonsteroidal anti-inflammatory therapy on the thermographic index. Ann. Rheum. Dis. 33, 353–356 (1974).
Ouellet, M. & Percival, M. D. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch. Biochem. Biophys. 387, 273–280 (2001).
Boutaud, O., Aronoff, D. M., Richardson, J. H., Marnett, L. J. & Oates, J. A. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc. Natl Acad. Sci. USA 99, 7130–7135 (2002).
Mielke, C. H. Jr. Comparative effects of aspirin and acetaminophen on hemostasis. Arch. Intern. Med. 141, 305–310 (1981).
Niemi, T. T., Backman, J. T., Syrjälä, M. T., Viinikka, L. U. & Rosenberg, P. H. Platelet dysfunction after intravenous ketorolac or propacetamol. Acta Anaesthesiol. Scand. 44, 69–74 (2000).
Munsterhjelm, E. et al. Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteers. Anesthesiology 103, 712–717 (2005).
Garcia Rodríguez, L. A. & Hernández-Díaz, S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res. 3, 98–101 (2001).
Rahme, E., Barkun, A., Nedjar, H., Gaugris, S. & Watson, D. Hospitalizations for upper and lower GI events associated with traditional NSAIDs and acetaminophen among the elderly in Quebec, Canada. Am. J. Gastroenterol. 103, 872–882 (2008).
Hinz, B. et al. Lumiracoxib inhibits cyclo-oxygenase 2 completely at the 50 mg dose: is liver toxicity avoidable by adequate dosing? Ann. Rheum. Dis. 68, 289–291 (2009).
Hammett, R. Urgent advice regarding management of patients taking lumiracoxib (Prexige). Australian Government Department of Ageing and Therapeutic Goods Administration [online], (2007).
Capone, M. L. et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation 109, 1468–1471 (2004).
Kearney, P. M. et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332, 1302–1308 (2006).
Goldstein, J. L. et al. Reduced incidence of upper gastrointestinal ulcer complications with the COX-2 selective inhibitor, valdecoxib. Aliment. Pharmacol. Ther. 20, 527–538 (2004).
Chan, F. K. et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N. Engl. J. Med. 347, 2104–2110 (2002).
Lai, K. C. et al. Celecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complications. Am. J. Med. 118, 1271–1278 (2005).
Goldstein, J. L. et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin. Gastroenterol. Hepatol. 3, 133–141 (2005).
Garcia Rodríguez, L. A., Tacconelli, S. & Patrignani, P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J. Am. Coll. Cardiol. 52, 1628–1636 (2008).
European Medicines Agency press release. European Medicines Agency recommends restricted use for piroxicam. European Medicines Agency [online], (2007).
Benson, M. D., Aldo-Benson, M. & Brandt, K. D. Synovial fluid concentrations of diclofenac in patients with rheumatoid arthritis or osteoarthritis. Semin. Arthritis Rheum. 15 (2 Suppl. 1), 65–67 (1985).
McCrea, J. D., Telford, A. M., Kaye, C. M. & Boyd, M. W. A comparison of plasma and synovial fluid profiles of standard and controlled-release formulations of ketoprofen in patients with rheumatoid arthritis. Curr. Med. Res. Opin. 10, 73–81 (1986).
Wagner, J. G., Albert, K. S., Szpunar, G. J. & Lockwood, G. F. Pharmacokinetics of ibuprofen in man IV: absorption and disposition. J. Pharmacokinet. Biopharm. 12, 381–399 (1984).
Author information
Authors and Affiliations
Contributions
K. Brune, B. Renner and B. Hinz researched the data for the article and K. Brune and B. Hinz provided a substantial contribution to discussions of the content. K. Brune and B. Hinz wrote the article and K. Brune, B. Renner and B. Hinz contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
K. Brune is a consultant for Aventis, Bayer, Merck and Novartis, and is on the speakers bureau and receives grant/research support from these companies. B. Renner receives grant/research support from GlaxoSmithKline and Merck Sharp & Dohme. B. Hinz is a consultant for Novartis, is on the speakers bureau for Merck Sharp & Dohme and Novartis, receives grant/research support from Bayer and Merck Sharp & Dohme, Mundipharma Pfizer and Sanofi.
Rights and permissions
About this article
Cite this article
Brune, K., Renner, B. & Hinz, B. Using pharmacokinetic principles to optimize pain therapy. Nat Rev Rheumatol 6, 589–598 (2010). https://doi.org/10.1038/nrrheum.2010.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.141
This article is cited by
-
Ibuprofen plasma concentration profile in deliberate ibuprofen overdose with circulatory depression treated with therapeutic plasma exchange: a case report
BMC Pharmacology and Toxicology (2017)
-
Clinical Pharmacology and Cardiovascular Safety of Naproxen
American Journal of Cardiovascular Drugs (2017)
-
NSAR in der Schmerztherapie
CME (2016)
-
Neuropathischer Schmerz
Der Schmerz (2015)
-
Pharmakologische Aspekte der Schmerzforschung in Deutschland
Der Schmerz (2015)