Abstract
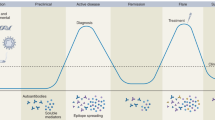
Biomarkers have an important influence on the clinical decision-making processes involved in diagnosis, assessment of disease activity, allocation of treatment, and determining prognosis. The clinical usefulness of a biomarker is dependant on demonstration of its validity. Ideally, biomarkers should provide information not available from currently available tests and should be tested as they would be used in clinical practice; however, potential biomarkers could be affected by many different clinical or patient variables—such as disease activity, therapeutic intervention, or the presence of comorbidities—and validation studies might not include all the design features that are required to ensure that the biomarker is a true measure of the clinical process it is intended to reflect. In this Review, we appraise studies that have been conducted to validate six promising new biomarkers for diagnosis, disease activity assessment, or prognosis in patients with systemic autoimmune diseases. We discuss the validity of these six biomarkers with particular reference to the features of the studies that lend weight to or distract from their findings. The intent of this discussion is to draw attention to elements of validation study design that should be considered when evaluating the robustness of a biomarker, which differ according to the marker's intended use.
Key Points
-
Biomarkers are important for making informed decisions in the clinic, including those concerning diagnosis and allocation of treatment, as well as for assessing disease activity and prognosis
-
The clinical usefulness of any biomarker depends on the demonstration of its validity for a particular purpose
-
In the validation of biomarkers intended to aid diagnosis, greater attention needs to be placed on controlling or adjusting for the demographic and clinical characteristics of the disease being studied
-
Longitudinal studies are essential in the assessment of validity for biomarkers relating to disease activity and prognosis
-
New biomarkers of the greatest value are those that provide information that cannot be gained from existing tests
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Atkinson, A. J. Jr et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Muñoz, A. & Gange, S. J. Methodological issues for biomarkers and intermediate outcomes in cohort studies. Epidemiol. Rev. 20, 29–42 (1998).
Illei, G. G., Tackey, E., Lapteva, L. & Lipsky, P. E. Biomarkers in systemic lupus erythematosus. I. General overview of biomarkers and their applicability. Arthritis Rheum. 50, 1709–1720 (2004).
Ward, M. M. Evaluative laboratory testing. Assessing tests that assess disease activity. Arthritis Rheum. 38, 1555–1563 (1995).
Tektonidou, M. G. & Ward, M. M. Validity of clinical associations of biomarkers in translational research studies: the case of systemic autoimmune diseases. Arthritis Res. Ther. 12, R179 (2010).
Sackett, D. L. & Haynes, R. B. The architecture of diagnostic research. BMJ 324, 539–541 (2002).
Baroni, S. S. et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354, 2667–2676 (2006).
Gabrielli, A. et al. Stimulatory autoantibodies to the PDGF receptor: a link to fibrosis in scleroderma and a pathway for novel therapeutic targets. Autoimmun. Rev. 7, 121–126 (2007).
Svegliati, S. et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood 110, 237–241 (2007).
Classen, J. F. et al. Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum. 60, 1137–1144 (2009).
Loizos, N. et al. Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor α in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum. 60, 1145–1151 (2009).
Gabrielli, A., Moroncini, G., Svegliati, S. & Avvedimento, E. V. Autoantibodies against the platelet-derived growth factor receptor in scleroderma: comment on the articles by Classen et al. and Loizos et al. Arthritis Rheum. 60, 3521–3522 (2009).
Balada, E. et al. Anti-PDGFR-α antibodies measured by non-bioactivity assays are not specific for systemic sclerosis. Ann. Rheum. Dis. 67, 1027–1029 (2008).
Kurasawa, K. et al. Autoantibodies against platelet-derived growth factor receptor alpha in patients with systemic lupus erythematosus. Mod. Rheumatol. 20, 458–465 (2010).
Becker, K. L., Nylén, E. S., White, J. C., Müller, B. & Snider, R. H. Jr. Clinical review 167: procalcitonin and the calcitonin gene family in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J. Clin. Endocrinol. Metab. 89, 1512–1525 (2004).
Simon, L., Gauvin, F., Amre, D. K., Saint-Louis, P. & Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin. Infect. Dis. 39, 206–217 (2004).
Tang, B. M., Eslick, G. D., Craig, J. C. & McLean, A. S. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect. Dis. 7, 210–217 (2007).
Eberhard, O. K. et al. Usefulness of procalcitonin for differentiation between activity of systemic autoimmune disease (systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis) and invasive bacterial infection. Arthritis Rheum. 40, 1250–1256 (1997).
Shin, K. C. et al. Serum procalcitonin measurement for detection of intercurrent infection in febrile patients with SLE. Ann. Rheum. Dis. 60, 988–989 (2001).
Lanoix, J. P. et al. Serum procalcitonin does not differentiate between infection and disease flare in patients with systemic lupus erythematosus. Lupus 20, 125–130 (2011).
Moosig, F., Csernok, E., Reinhold-Keller, E., Schmitt, W. & Gross, W. L. Elevated procalcitonin levels in active Wegener's granulomatosis. J. Rheumatol. 25, 1531–1533 (1998).
Schwenger, V., Sis, J., Breitbart, A. & Andrassy, K. CRP levels in autoimmune disease can be specified by measurement of procalcitonin. Infection 26, 274–276 (1998).
Zycinska, K., Wardyn, K. A., Zielonka, T. M., Tyszko, P. & Straburzynski, M. Procalcitonin as an indicator of systemic response to infection in active pulmonary Wegener's granulomatosis. J. Physiol. Pharmacol. 59 (Suppl. 6), 839–844 (2008).
Tamaki, K. et al. Diagnostic accuracy of serum procalcitonin concentrations for detecting systemic bacterial infection in patients with systemic autoimmune diseases. J. Rheumatol. 35, 114–119 (2008).
Scirè, C. A. et al. Diagnostic value of procalcitonin measurement in febrile patients with systemic autoimmune diseases. Clin. Exp. Rheumatol. 24, 123–128 (2006).
Chen, D. Y. et al. Diagnostic value of procalcitonin for differentiation between bacterial infection and non-infectious inflammation in febrile patients with active adult-onset Still's disease. Ann. Rheum. Dis. 68, 1074–1075 (2009).
Griffiths, B., Mosca, M. & Gordon, C. Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices. Best Pract. Res. Clin. Rheumatol. 19, 685–708 (2005).
Liu, C. C. & Ahearn, J. M. The search for lupus biomarkers. Best Pract. Res. Clin. Rheumatol. 23, 507–523 (2009).
Kirou, K. A. et al. Coordinate overexpression of interferon-α-induced genes in systemic lupus erythematosus. Arthritis Rheum. 50, 3958–3967 (2004).
Baechler, E. C., Gregersen, P. K. & Behrens, T. W. The emerging role of interferon in human systemic lupus erythematosus. Curr. Opin. Immunol. 16, 801–807 (2004).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Kirou, K. A. et al. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Feng, X. et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 54, 2951–2962 (2006).
Nikpour, M., Dempsey, A. A., Urowitz, M. B., Gladman, D. D. & Barnes, D. A. Association of a gene expression profile from whole blood with disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 67, 1069–1075 (2008).
Landolt-Marticorena, C. et al. Lack of association between the interferon-α signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 68, 1440–1446 (2009).
Kaneko H. et al. Circulating levels of beta-chemokines in systemic lupus erythematosus. J. Rheumatol. 26, 568–573 (1999).
Lit, L. C., Wong, C. K., Tam, L. S., Li, E. K. & Lam, C. W. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 65, 209–215 (2006).
Fu, Q. et al. Association of elevated transcript levels of interferon-inducible chemokines with disease activity and organ damage in systemic lupus erythematosus patients. Arthritis Res. Ther. 10, R112 (2008).
Narumi, S., Takeuchi, T., Kobayashi, Y. & Konishi, K. Serum levels of IFN-inducible protein-10 relating to the activity of systemic lupus erythematosus. Cytokine 12, 1561–1565 (2000).
Bauer, J. W. et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 3, e491 (2006).
Vilá, L. M. et al. Association of serum MIP-1α, MIP-1β, and RANTES with clinical manifestations, disease activity, and damage accrual in systemic lupus erythematosus. Clin. Rheumatol. 26, 718–722 (2007).
Bauer, J. W. et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 60, 3098–3107 (2009).
Buyon, J. P., Tamerius, J., Belmont, H. M. & Abramson, S. B. Assessment of disease activity and impending flare in patients with systemic lupus erythematosus. Comparison of the use of complement split products and conventional measurements of complement. Arthritis Rheum. 35, 1028–1037 (1992).
Calano, S. J. et al. Cell-bound complement activation products (CB-CAPs) as a source of lupus biomarkers. Adv. Exp. Med. Biol. 586, 381–390 (2006).
Manzi, S., Ahearn, J. M. & Salmon, J. New insights into complement: a mediator of injury and marker of disease activity in systemic lupus erythematosus. Lupus 13, 298–303 (2004).
Manzi, S. et al. Measurement of erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. Arthritis Rheum. 50, 3596–3604 (2004).
Singh, V., Mahoney, J. A. & Petri, M. Erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. J. Rheumatol. 35, 1989–1993 (2008).
Yang, D. H., Chang, D. M., Lai, J. H., Lin, F. H. & Chen, C. H. Usefulness of erythrocyte-bound C4d as a biomarker to predict disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 48, 1083–1087 (2009).
Kao, A. H. et al. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis Rheum. 62, 837–844 (2010).
Liu, C. C. et al. Reticulocytes bearing C4d as biomarkers of disease activity for systemic lupus erythematosus. Arthritis Rheum. 52, 3087–3099 (2005).
Navratil, J. S. et al. Platelet C4d is highly specific for systemic lupus erythematosus. Arthritis Rheum. 54, 670–674 (2006).
Garnero, P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction, and therapy monitoring. Mol. Diagn. Ther. 12, 157–170 (2008).
Garnero, P., Rousseau, J. C. & Delmas, P. D. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 43, 953–968 (2000).
Garnero, P., Jouvenne, P., Buchs, N., Delmas, P. D. & Miossec, P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone 24, 381–385 (1999).
Garnero, P. et al. Association of baseline levels of markers of bone and cartilage degradation with long-term prognosis of joint damage in patients with early arthritis. The COBRA study. Arthritis Rheum. 46, 2847–2856 (2002).
Landewé, R. et al. Markers for type II collagen breakdown predict the effect of disease-modifying treatment on long-term radiographic progression in patients with rheumatoid arthritis. Arthritis Rheum. 50, 1390–1399 (2004).
Jansen, L. M. et al. Serological bone markers and joint damage in early polyarthritis. J. Rheumatol. 31, 1491–1496 (2004).
Forsblad d'Elia, H. et al. Hormone replacement therapy, calcium and vitamin D3 versus calcium and vitamin D3 alone decreases markers of cartilage and bone metabolism in rheumatoid arthritis: a randomized controlled trial [ISRCTN46523456]. Arthritis Res. Ther. 6, R457–R468 (2004).
Syversen, S. W. et al. Cartilage and bone biomarkers in rheumatoid arthritis: prediction of 10-year radiographic progression. J. Rheumatol. 36, 266–272 (2009).
Wisłowska, M., Jakubicz, D., Stepień, K. & Cicha, M. Serum concentrations of formation (PINP) and resorption (Ctx) bone turnover markers in rheumatoid arthritis. Rheumatol. Int. 29, 1403–1409 (2009).
van Tuyl, L. H. et al. Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann. Rheum. Dis. 69, 1623–1628 (2010).
Christgau, S. et al. Collagen type II C-telopeptide fragments as an index of cartilage degradation. Bone 29, 209–215 (2001).
Garnero, P., Gineyts, E., Christgau, S., Finck, B. & Delmas, P. D. Association of baseline levels of urinary glucosyl-galactosyl-pyridinoline and type II collagen C-telopeptide with progression of joint destruction in patients with early rheumatoid arthritis. Arthritis Rheum. 46, 21–30 (2002).
Young-Min, S. et al. Biomarkers predict radiographic progression in early rheumatoid arthritis and perform well compared to traditional markers. Arthritis Rheum. 56, 3236–3247 (2007).
Marotte, H., Gineyts, E., Miossec, P. & Delmas, P. D. Effects of infliximab therapy on biological markers of synovium activity and cartilage breakdown in patients with rheumatoid arthritis. Ann. Rheum. Dis. 68, 1197–1200 (2009).
Hashimoto, J. et al. A combination of biochemical markers of cartilage and bone turnover, radiographic damage and body mass index to predict the progression of joint destruction in patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs. Mod. Rheumatol. 19, 273–282 (2009).
Christensen, A. F. et al. Differential association of the N-propeptide of collagen IIA (PIIANP) and collagen II C-telopeptide (CTX-II) with synovitis and erosions in early and longstanding rheumatoid arthritis. Clin. Exp. Rheumatol. 27, 307–314 (2009).
Christensen, A. F. et al. Uncoupling of collagen II metabolism in newly diagnosed, untreated rheumatoid arthritis is linked to inflammation and antibodies against cyclic citrullinated peptides. J. Rheumatol. 37, 1113–1120 (2010).
Maksymowych, W. P. et al. Reappraisal of OMERACT 8 draft validation criteria for a soluble biomarker reflecting structural damage endpoints in rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis: the OMERACT 9 v2 criteria. J. Rheumatol. 36, 1785–1791 (2009).
Maksymowych, W. P. et al. Proposal for levels of evidence scheme for validation of a soluble biomarker reflecting damage endpoints in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, and recommendations for study design. J. Rheumatol. 36, 1792–1799 (2009).
Acknowledgements
This work was supported in part by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, USA.
Author information
Authors and Affiliations
Contributions
M. G. Tektonidou and M. M. Ward contributed equally to researching the data for the article, discussing the content, writing the article and reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tektonidou, M., Ward, M. Validation of new biomarkers in systemic autoimmune diseases. Nat Rev Rheumatol 7, 708–717 (2011). https://doi.org/10.1038/nrrheum.2011.157
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.157
This article is cited by
-
The diagnostic role of the systemic inflammation index in patients with immunological diseases: a systematic review and meta-analysis
Clinical and Experimental Medicine (2024)
-
Bone mineral density and explanatory factors in children and adults with juvenile dermatomyositis at long term follow-up; a cross sectional study
Pediatric Rheumatology (2021)
-
Circulating S100 proteins effectively discriminate SLE patients from healthy controls: a cross-sectional study
Rheumatology International (2019)
-
Review of biomarkers in systemic juvenile idiopathic arthritis: helpful tools or just playing tricks?
Arthritis Research & Therapy (2016)
-
Liquid biopsies in patients with diffuse glioma
Acta Neuropathologica (2015)