Key Points
-
A diagnosis of juvenile idiopathic arthritis (JIA) routinely results in many years of immunosuppressive therapy, administered daily or weekly as appropriate for the therapeutic agent
-
Validated and reliable clinical, biological or molecular markers that predict treatment efficacy and safety are currently lacking
-
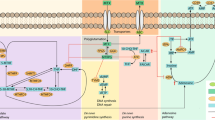
Candidate-gene and genome-wide pharmacogenetic studies in JIA have identified single nucleotide polymorphisms (SNPs) and gene regions potentially associated with toxicity or response to methotrexate (MTX) and etanercept
-
Associations of SNPs and gene regions with the MTX and etanercept pathways have not been replicated or shown consistency
-
Studies in children provide the unique opportunity to understand ontogeny processes in addition to disease and treatment associations
-
Access to large, well-documented cohorts of patients with JIA and advanced molecular techniques might facilitate the identification of novel pathways and of patients likely to respond to a specific drug
Abstract
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic condition in childhood, with many children requiring immunomodulatory therapies for many years following diagnosis. A considerable proportion of children experience therapeutic inefficacy or substantial adverse effects, or both, but a lack of reliable clinical indicators and biomarkers to predict treatment response prevents optimization of existing therapies. The identification of valid candidate gene variants involved in the pathways of methotrexate and etanercept, the most commonly used medications in JIA, has seen little success to date. The limited success of these studies is possibly due to the presence of confounding variables in the study populations, the heterogeneity of outcome parameters used to determine treatment response and the small number of candidate gene variants analysed. The first genome-wide pharmacogenetic study in JIA has identified gene regions of particular biological interest, but these findings require validation. Moreover, epigenetic mechanisms as well as ontogeny processes might be additional factors influencing drug responses. Access to large, well-documented JIA cohorts and the rapid development of advanced genome analytics is ushering in a personalized approach to treatment. The discovery of new pharmacogenomic biomarkers and systems pathways can provide new drug targets and predictive tools for improved drug response and fewer adverse drug reactions in JIA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Andersson Gäre, B. Juvenile arthritis—who gets it, where and when? A review of current data on incidence and prevalence. Clin. Exp. Rheumatol. 17, 367–374 (1999).
Petty, R. E. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 31, 390–392 (2004).
Horneff, G. Juvenile Idiopathic Arthritis (Uni. Med. Verlag, 2014).
Adib, N., Silman, A. & Thomson, W. Outcome following onset of juvenile idiopathic inflammatory arthritis: I. frequency of different outcomes. Rheumatology (Oxford) 44, 995–1001 (2005).
Ringold, S. & Wallace, C. A. Measuring clinical response and remission in juvenile idiopathic arthritis. Curr. Opin. Rheumatol. 19, 471–476 (2007).
Wallace, C. A., Ravelli, A., Huang, B. & Giannini, E. H. Preliminary validation of clinical remission criteria using the OMERACT filter for select categories of juvenile idiopathic arthritis. J. Rheumatol. 33, 789–795 (2006).
Wallace, C. A., Ruperto, N. & Giannini, E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J. Rheumatol. 31, 2290–2294 (2004).
Wallace, C. A., Huang, B., Bandeira, M., Ravelli, A. & Giannini, E. H. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum. 52, 3554–3562 (2005).
Giannini, E. H. et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 40, 1202–1209 (1997).
Consolaro, A. et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 61, 658–666 (2009).
Wallace, C. A., Giannini, E. H., Huang, B., Itert, L. & Ruperto, N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res. (Hoboken) 63, 929–936 (2011).
Filocamo, G. et al. A new approach to clinical care of juvenile idiopathic arthritis: the Juvenile Arthritis Multidimensional Assessment Report. J. Rheumatol. 38, 938–953 (2011).
McErlane, F., Beresford, M. W., Baildam, E. M., Thomson, W. & Hyrich, K. L. Recent developments in disease activity indices and outcome measures for juvenile idiopathic arthritis. Rheumatology (Oxford) 52, 1941–1951 (2013).
Dueckers, G. et al. Evidence and consensus based GKJR guidelines for the treatment of juvenile idiopathic arthritis. Clin. Immunol. 142, 176–193 (2012).
Ringold, S. et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 65, 2499–2512 (2013).
Beukelman, T. et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. (Hoboken) 63, 465–482 (2011).
van der Heijden, J. W., Dijkmans, B. A., Scheper, R. J. & Jansen, G. Drug insight: resistance to methotrexate and other disease-modifying antirheumatic drugs—from bench to bedside. Nat. Clin. Pract. Rheumatol. 3, 26–34 (2007).
Maneiro, J. R., Salgado, E. & Gomez-Reino, J. J. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated inflammatory conditions: systematic review and meta-analysis. JAMA Intern. Med. 173, 1416–1428 (2013).
Vincent, F. B. et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann. Rheum. Dis. 72, 165–178 (2013).
Altman, R. B. Genetic sequence data for pharmacogenomics. Curr. Opin. Drug Discov. Devel. 6, 297–303 (2003).
McLeod, H. L. Drug pathways: moving beyond single gene pharmacogenetics. Pharmacogenomics 5, 139–141 (2004).
Eichelbaum, M., Altman, R. B., Ratain, M. & Klein, T. E. New feature: pathways and important genes from PharmGKB. Pharmacogenet. Genomics 19, 403 (2009).
Pharmacogenomics Knowledge Base. PharmGKB [online], (2014).
Thorn, C. F., Klein, T. E. & Altman, R. B. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol. Biol. 1015, 311–320 (2013).
Ramanan, A. V., Whitworth, P. & Baildam, E. M. Use of methotrexate in juvenile idiopathic arthritis. Arch. Dis. Child. 88, 197–200 (2003).
Hashkes, P. J. & Laxer, R. M. Update on the medical treatment of juvenile idiopathic arthritis. Curr. Rheumatol. Rep. 8, 450–458 (2006).
Dervieux, T. et al. HPLC determination of erythrocyte methotrexate polyglutamates after low-dose methotrexate therapy in patients with rheumatoid arthritis. Clin. Chem. 49, 1632–1641 (2003).
Ranganathan, P. & McLeod, H. L. Methotrexate pharmacogenetics: the first step toward individualized therapy in rheumatoid arthritis. Arthritis Rheum. 54, 1366–1377 (2006).
Tian, H. & Cronstein, B. N. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull. NYU Hosp. Jt Dis. 65, 168–173 (2007).
Angelis-Stoforidis, P., Vajda, F. J. & Christophidis, N. Methotrexate polyglutamate levels in circulating erythrocytes and polymorphs correlate with clinical efficacy in rheumatoid arthritis. Clin. Exp. Rheumatol. 17, 313–320 (1999).
Dervieux, T., Meshkin, B. & Neri, B. Pharmacogenetic testing: proofs of principle and pharmacoeconomic implications. Mutat. Res. 573, 180–194 (2005).
Montesinos, M. C. et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 48, 240–247 (2003).
Montesinos, M. C. et al. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 56, 1440–1445 (2007).
Mikkelsen, T. S. et al. PharmGKB summary: methotrexate pathway. Pharmacogenet. Genomics 21, 679–686 (2011).
Bannwarth, B., Pehourcq, F., Schaeverbeke, T. & Dehais, J. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin. Pharmacokinet. 30, 194–210 (1996).
Cronstein, B. N. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev. 57, 163–172 (2005).
Morgan, S. L. & Baggott, J. E. Folate supplementation during methotrexate therapy for rheumatoid arthritis. Clin. Exp. Rheumatol. 28 (Suppl. 61), S102–S109 (2010).
Chan, E. S., Fernandez, P. & Cronstein, B. N. Adenosine in inflammatory joint diseases. Purinergic Signal. 3, 145–152 (2007).
Cronstein, B. N., Naime, D. & Ostad, E. The antiinflammatory effects of methotrexate are mediated by adenosine. Adv. Exp. Med. Biol. 370, 411–416 (1994).
Cronstein, B. N., Naime, D. & Ostad, E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Invest. 92, 2675–2682 (1993).
Cronstein, B. N. The pharmacology of antiinflammatory agents: a new paradigm. Mt Sinai J. Med. 60, 209–217 (1993).
Cronstein, B. N. Molecular mechanism of methotrexate action in inflammation. Inflammation 16, 411–423 (1992).
Montesinos, M. C., Desai, A. & Cronstein, B. N. Suppression of inflammation by low-dose methotrexate is mediated by adenosine A2A receptor but not A3 receptor activation in thioglycollate-induced peritonitis. Arthritis Res. Ther. 8, R53 (2006).
Giannini, E. H. et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.–U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children's Study Group. N. Engl. J. Med. 326, 1043–1049 (1992).
Bulatovic, M. et al. High prevalence of methotrexate intolerance in juvenile idiopathic arthritis: development and validation of a methotrexate intolerance severity score. Arthritis Rheum. 63, 2007–2013 (2011).
Mulligan, K. et al. Mothers' reports of the difficulties that their children experience in taking methotrexate for Juvenile Idiopathic Arthritis and how these impact on quality of life. Pediatr. Rheumatol. Online J. 11, 23 (2013).
Owen, S. A. et al. MTHFR gene polymorphisms and outcome of methotrexate treatment in patients with rheumatoid arthritis: analysis of key polymorphisms and meta-analysis of C677T and A1298C polymorphisms. Pharmacogenomics J. 13, 137–147 (2013).
Ansari, M. & Krajinovic, M. Pharmacogenomics in cancer treatment defining genetic bases for inter-individual differences in responses to chemotherapy. Curr. Opin. Pediatr. 19, 15–22 (2007).
Rozen, R. Molecular genetics of methylenetetrahydrofolate reductase deficiency. J. Inherit. Metab. Dis. 19, 589–594 (1996).
Haagsma, C. J. et al. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann. Rheum. Dis. 58, 79–84 (1999).
van Ede, A. E. et al. The C677T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis Rheum. 44, 2525–2530 (2001).
Kumagai, K., Hiyama, K., Oyama, T., Maeda, H. & Kohno, N. Polymorphisms in the thymidylate synthase and methylenetetrahydrofolate reductase genes and sensitivity to the low-dose methotrexate therapy in patients with rheumatoid arthritis. Int. J. Mol. Med. 11, 593–600 (2003).
Berkun, Y. et al. Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Ann. Rheum. Dis. 63, 1227–1231 (2004).
Urano, W. et al. Polymorphisms in the methylenetetrahydrofolate reductase gene were associated with both the efficacy and the toxicity of methotrexate used for the treatment of rheumatoid arthritis, as evidenced by single locus and haplotype analyses. Pharmacogenetics 12, 183–190 (2002).
James, H. M. et al. Common polymorphisms in the folate pathway predict efficacy of combination regimens containing methotrexate and sulfasalazine in early rheumatoid arthritis. J. Rheumatol. 35, 562–571 (2008).
Zeng, Q. Y., Wang, Y. K., Xiao, Z. Y. & Chen, S. B. Pharmacogenetic study of 5, 10-methylenetetrahydrofolate reductase C677T and thymidylate synthase 3R/2R gene polymorphisms and methotrexate-related toxicity in Chinese Han patients with inflammatory arthritis. Ann. Rheum. Dis. 67, 1193–1194 (2008).
Aggarwal, P., Naik, S., Mishra, K. P., Aggarwal, A. & Misra, R. Correlation between methotrexate efficacy & toxicity with C677T polymorphism of the methylenetetrahydrofolate gene in rheumatoid arthritis patients on folate supplementation. Indian J. Med. Res. 124, 521–526 (2006).
Kurzawski, M., Pawlik, A., Safranow, K., Herczynska, M. & Drozdzik, M. 677C>T and 1298A>C MTHFR polymorphisms affect methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics 8, 1551–1559 (2007).
Spyridopoulou, K. P., Dimou, N. L., Hamodrakas, S. J. & Bagos, P. G. Methylene tetrahydrofolate reductase gene polymorphisms and their association with methotrexate toxicity: a meta-analysis. Pharmacogenet. Genomics 22, 117–133 (2012).
Taniguchi, A. et al. Validation of the associations between single nucleotide polymorphisms or haplotypes and responses to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a proposal for prospective pharmacogenomic study in clinical practice. Pharmacogenet. Genomics 17, 383–390 (2007).
Todorovic, Z. et al. Homocysteine serum levels and MTHFR C677T genotype in patients with Parkinson's disease, with and without levodopa therapy. J. Neurol. Sci. 248, 56–61 (2006).
Herrlinger, K. R. et al. The pharmacogenetics of methotrexate in inflammatory bowel disease. Pharmacogenet. Genomics 15, 705–711 (2005).
Frosst, P. et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 10, 111–113 (1995).
Chan, E. S. & Cronstein, B. N. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 4, 266–273 (2002).
Schmeling, H., Biber, D., Heins, S. & Horneff, G. Influence of methylenetetrahydrofolate reductase polymorphisms on efficacy and toxicity of methotrexate in patients with juvenile idiopathic arthritis. J. Rheumatol. 32, 1832–1836 (2005).
van der Put, N. M. et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am. J. Hum. Genet. 62, 1044–1051 (1998).
Weisberg, I., Tran, P., Christensen, B., Sibani, S. & Rozen, R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 64, 169–172 (1998).
Tukova, J. et al. 677TT genotype is associated with elevated risk of methotrexate (MTX) toxicity in juvenile idiopathic arthritis: treatment outcome, erythrocyte concentrations of MTX and folates, and MTHFR polymorphisms. J. Rheumatol. 37, 2180–2186 (2010).
Yanagimachi, M. et al. Influence of polymorphisms within the methotrexate pathway genes on the toxicity and efficacy of methotrexate in patients with juvenile idiopathic arthritis. Br. J. Clin. Pharmacol. 71, 237–243 (2011).
Panetta, J. C., Wall, A., Pui, C. H., Relling, M. V. & Evans, W. E. Methotrexate intracellular disposition in acute lymphoblastic leukemia: a mathematical model of gamma-glutamyl hydrolase activity. Clin. Cancer Res. 8, 2423–2429 (2002).
Becker, M. L. et al. The effect of genotype on methotrexate polyglutamate variability in juvenile idiopathic arthritis and association with drug response. Arthritis Rheum. 63, 276–285 (2011).
Albers, H. M. et al. Time to treatment as an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Rheum. 61, 46–51 (2009).
Moncrieffe, H. et al. Generation of novel pharmacogenomic candidates in response to methotrexate in juvenile idiopathic arthritis: correlation between gene expression and genotype. Pharmacogenet. Genomics 20, 665–676 (2010).
Delerive, P., Fruchart, J. C. & Staels, B. Peroxisome proliferator-activated receptors in inflammation control. J. Endocrinol. 169, 453–459 (2001).
Jansen, S., Cashman, K., Thompson, J. G., Pantaleon, M. & Kaye, P. L. Glucose deprivation, oxidative stress and peroxisome proliferator-activated receptor-alpha (PPARA) cause peroxisome proliferation in preimplantation mouse embryos. Reproduction 138, 493–505 (2009).
Hinks, A. et al. Association of the 5-aminoimidazole-4-carboxamide ribonucleotide transformylase gene with response to methotrexate in juvenile idiopathic arthritis. Ann. Rheum. Dis. 70, 1395–1400 (2011).
de Rotte, M. C. et al. ABCB1 and ABCC3 gene polymorphisms are associated with first-year response to methotrexate in juvenile idiopathic arthritis. J. Rheumatol. 39, 2032–2040 (2012).
Ott, C. J. et al. Intronic enhancers coordinate epithelial-specific looping of the active CFTR locus. Proc. Natl Acad. Sci. USA 106, 19934–19939 (2009).
Conseil, G., Deeley, R. G. & Cole, S. P. Polymorphisms of MRP1 (ABCC1) and related ATP-dependent drug transporters. Pharmacogenet. Genomics 15, 523–533 (2005).
Bulatovic, M. et al. Prediction of clinical non-response to methotrexate treatment in juvenile idiopathic arthritis. Ann. Rheum. Dis. 71, 1484–1489 (2012).
Cobb, J. et al. Genome-wide data reveal novel genes for methotrexate response in a large cohort of juvenile idiopathic arthritis cases. Pharmacogenomics J. http://dx.doi.org/10.1038/tpj.2014.3/.
Li, X., Thyssen, G., Beliakoff, J. & Sun, Z. The novel PIAS-like protein hZimp10 enhances Smad transcriptional activity. J. Biol. Chem. 281, 23748–23756 (2006).
Ellinghaus, D. et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am. J. Hum. Genet. 90, 636–647 (2012).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Patsopoulos, N. A. et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann. Neurol. 70, 897–912 (2011).
Horneff, G. Update on biologicals for treatment of juvenile idiopathic arthritis. Expert Opin.Biol. Ther. 13, 361–376 (2013).
Shenoi, S. & Wallace, C. A. Tumor necrosis factor inhibitors in the management of juvenile idiopathic arthritis: an evidence-based review. Paediatr. Drugs 12, 367–377 (2010).
Hayward, K. & Wallace, C. A. Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res. Ther. 11, 216 (2009).
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117, 244–279 (2008).
Horneff, G. et al. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann. Rheum. Dis. 63, 1638–1644 (2004).
Anink, J. et al. Treatment choices of paediatric rheumatologists for juvenile idiopathic arthritis: etanercept or adalimumab? Rheumatology (Oxford) 52, 1674–1679 (2013).
Horneff, G. et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann. Rheum. Dis. 73, 1114–1122 (2014).
Kohno, T., Tam, L. T., Stevens, S. R. & Louie, J. S. Binding characteristics of tumor necrosis factor receptor-Fc fusion proteins vs anti-tumor necrosis factor mAbs. J. Investig. Dermatol. Symp. Proc. 12, 5–8 (2007).
Giannini, E. H. et al. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 60, 2794–2804 (2009).
Lovell, D. J. et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 58, 1496–1504 (2008).
Schmeling, H. & Horneff, G. Tumour necrosis factor alpha promoter polymorphisms and etanercept therapy in juvenile idiopathic arthritis. Rheumatol. Int. 27, 383–386 (2007).
Basic, J. et al. Etanercept reduces matrix metalloproteinase-9 level in children with polyarticular juvenile idiopathic arthritis and TNF-alpha-308GG genotype. J. Physiol. Biochem. 66, 173–180 (2010).
Cimaz, R. et al. IL1 and TNF gene polymorphisms in patients with juvenile idiopathic arthritis treated with TNF inhibitors. Ann. Rheum. Dis. 66, 900–904 (2007).
Allen, R. D. Polymorphism of the human TNF-alpha promoter—random variation or functional diversity? Mol. Immunol. 36, 1017–1027 (1999).
Makhatadze, N. J. Tumor necrosis factor locus: genetic organisation and biological implications. Hum. Immunol. 59, 571–579 (1998).
Kacevska, M., Ivanov, M. & Ingelman-Sundberg, M. Perspectives on epigenetics and its relevance to adverse drug reactions. Clin. Pharmacol. Ther. 89, 902–907 (2011).
Gomez, A. & Ingelman-Sundberg, M. Pharmacoepigenetic aspects of gene polymorphism on drug therapies: effects of DNA methylation on drug response. Expert Rev. Clin. Pharmacol. 2, 55–65 (2009).
Ellis, J. A. et al. Genome-scale case-control analysis of CD4+ T-cell DNA methylation in juvenile idiopathic arthritis reveals potential targets involved in disease. Clin. Epigenetics 4, 20 (2012).
Meda, F., Folci, M., Baccarelli, A. & Selmi, C. The epigenetics of autoimmunity. Cell. Mol. Immunol. 8, 226–236 (2011).
Balada, E., Ordi-Ros, J. & Vilardell-Tarres, M. DNA methylation and systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 1108, 127–136 (2007).
Javierre, B. M. et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 20, 170–179 (2010).
Liu, C. C. et al. Global DNA methylation, DNMT1, and MBD2 in patients with systemic lupus erythematosus. Lupus 20, 131–136 (2011).
Nakano, K., Whitaker, J. W., Boyle, D. L., Wang, W. & Firestein, G. S. DNA methylome signature in rheumatoid arthritis. Ann. Rheum. Dis. 72, 110–117 (2013).
De Santis, M. & Selmi, C. The therapeutic potential of epigenetics in autoimmune diseases. Clin. Rev. Allergy Immunol. 42, 92–101 (2012).
Wagner-Weiner, L. Pediatric rheumatology for the adult rheumatologist. J. Clin. Rheumatol. 14, 109–119 (2008).
Heiligenhaus, A., Heinz, C., Edelsten, C., Kotaniemi, K. & Minden, K. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul. Immunol. Inflamm. 21, 180–191 (2013).
Angeles-Han, S. T. et al. Risk markers of juvenile idiopathic arthritis-associated uveitis in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. J. Rheumatol. 40, 2088–2096 (2013).
Coulson, E. J., Hanson, H. J. & Foster, H. E. What does an adult rheumatologist need to know about juvenile idiopathic arthritis? Rheumatology (Oxford) http://dx.doi.org/10.1093/rheumatology/keu257/.
Ward, R. M. & Kauffman, R. Future of pediatric therapeutics: reauthorization of BPCA and PREA. Clin. Pharmacol. Ther. 81, 477–479 (2007).
Steinbrook, R. Testing medications in children. N. Engl. J. Med. 347, 1462–1470 (2002).
Hines, R. N. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol. Ther. 118, 250–267 (2008).
Husain, A., Loehle, J. A. & Hein, D. W. Clinical pharmacogenetics in pediatric patients. Pharmacogenomics 8, 1403–1411 (2007).
Goldman, J., Becker, M. L., Jones, B., Clements, M. & Leeder, J. S. Development of biomarkers to optimize pediatric patient management: what makes children different? Biomark. Med. 5, 781–794 (2011).
Alcorn, J. & McNamara, P. J. Ontogeny of hepatic and renal systemic clearance pathways in infants: part II. Clin. Pharmacokinet. 41, 1077–1094 (2002).
Alcorn, J. & McNamara, P. J. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin. Pharmacokinet. 41, 959–998 (2002).
Fanos, V., Antonucci, R. & Atzori, L. Metabolomics in the developing infant. Curr. Opin. Pediatr. http://dx.doi.org/10.1097/MOP.0b013e328363ec8b/.
Mussap, M., Antonucci, R., Noto, A. & Fanos, V. The role of metabolomics in neonatal and pediatric laboratory medicine. Clin. Chim. Acta 426, 127–138 (2013).
Kearns, G. L. et al. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 349, 1157–1167 (2003).
Relling, M. V. et al. Granulocyte colony-stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood 101, 3862–3867 (2003).
Hartford, C. et al. Genome scan implicates adhesion biological pathways in secondary leukemia. Leukemia 21, 2128–2136 (2007).
Carleton, B. Demonstrating utility of pharmacogenetics in pediatric populations: methodological considerations. Clin. Pharmacol. Ther. 88, 757–759 (2010).
Becker, M. L. & Leeder, J. S. Identifying genomic and developmental causes of adverse drug reactions in children. Pharmacogenomics 11, 1591–1602 (2010).
Isaacs, J. D. & Ferraccioli, G. The need for personalised medicine for rheumatoid arthritis. Ann. Rheum. Dis. 70, 4–7 (2011).
Author information
Authors and Affiliations
Contributions
H.S. researched data and wrote the manuscript. H.S., G.H., S.M.B. and M.J.F. contributed substantially for the discussion of content and in reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.S. has received funding for industry-driven clinical trials, advisory board membership and honourara from AbbVie, Pfizer, Roche and UCB. G.H. has received funding and speaker fees from AbbVie, Pfizer, Novartis and Roche. S.B. and M.J.F. declare no competing interests.
Rights and permissions
About this article
Cite this article
Schmeling, H., Horneff, G., Benseler, S. et al. Pharmacogenetics: can genes determine treatment efficacy and safety in JIA?. Nat Rev Rheumatol 10, 682–690 (2014). https://doi.org/10.1038/nrrheum.2014.140
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.140
This article is cited by
-
Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting
Pediatric Rheumatology (2018)
-
In the Pursuit of Methotrexate Treatment Response Biomarker in Juvenile Idiopathic Arthritis—Are We Getting Closer to Personalised Medicine?
Current Rheumatology Reports (2017)