Key Points
-
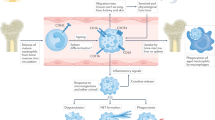
Eosinophils are granulocytic cells that secrete a wide variety of cytokines, chemokines and other mediators that probably have direct and indirect roles in the pathogenesis of eosinophilic granulomatosis with polyangiitis (EGPA)
-
The clinical manifestations of eosinophil accumulation and activation include tissue fibrosis, thrombosis, and allergic inflammation but depend, to a large degree, on the particular tissue involved
-
Although data is scarce, eosinophils seem to have a central role in disease pathogenesis in all three clinical stages of EGPA
-
Clinical trials using novel therapeutic strategies that specifically target eosinophils are helping define the role of eosinophils in the pathogenesis of EGPA
Abstract
Eosinophils are multifunctional granular leukocytes that are implicated in the pathogenesis of a wide variety of disorders, including asthma, helminth infection, and rare hypereosinophilic syndromes. Although peripheral and tissue eosinophilia can be a feature of many types of small-vessel and medium-vessel vasculitis, the role of eosinophils has been best studied in eosinophilic granulomatosis with polyangiitis (EGPA), where eosinophils are a characteristic finding in all three clinical stages of the disorder. Whereas numerous studies have demonstrated an association between the presence of eosinophils and markers of eosinophil activation in the blood and tissues of patients with EGPA, the precise role of eosinophils in disease pathogenesis has been difficult to ascertain owing to the complexity of the disease process. In this regard, results of clinical trials using novel agents that specifically target eosinophils are providing the first direct evidence of a central role of eosinophils in EGPA. This Review focuses on the aspects of eosinophil biology most relevant to the pathogenesis of vasculitis and provides an update of current knowledge regarding the role of eosinophils in EGPA and other vasculitides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Masi, A. T. et al. The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 33, 1094–1100 (1990).
Jennette, J. C. et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 37, 187–192 (1994).
Gleich, G. J. & Adolphson, C. R. The eosinophilic leukocyte: structure and function. Adv. Immunol. 39, 177–253 (1986).
Mullick, F. G., McAllister, H. A. Jr, Wagner, B. M. & Fenoglio, J. J. Jr. Drug related vasculitis. Clinicopathologic correlations in 30 patients. Hum. Pathol. 10, 313–325 (1979).
Robinowitz, M., Virmani, R. & McAllister, H. A. J. Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am. J. Med. 72, 923–928 (1982).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
John, A. E., Thomas, M. S., Berlin, A. A. & Lukacs, N. W. Temporal production of CCL28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway inflammation. Am. J. Pathol. 166, 345–353 (2005).
Hogan, S. P. et al. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy 38, 709–750 (2008).
Moqbel, R. & Lacy, P. New concepts in effector functions of eosinophil cytokines. Clin. Exp. Allergy 30, 1667–1671 (2000).
Walsh, G. M. Eosinophil granule proteins and their role in disease. Curr. Opin. Hematol. 8, 28–33 (2001).
Dvorak, A. M., Letourneau, L., Login, G. R., Weller, P. F. & Ackerman, S. J. Ultrastructural localization of the Charcot–Leyden crystal protein (lysophospholipase) to a distinct crystalloid-free granule population in mature human eosinophils. Blood 72, 150–158 (1988).
Melo, R. C., Paganoti, G. F., Dvorak, A. M. & Weller, P. F. The internal architecture of leukocyte lipid body organelles captured by three-dimensional electron microscopy tomography. PLoS ONE 8, e59578 (2013).
Melo, R. C. et al. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab. Invest. 89, 769–781 (2009).
Melo, R. C., Perez, S. A., Spencer, L. A., Dvorak, A. M. & Weller, P. F. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic 6, 866–879 (2005).
Spencer, L. A. et al. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc. Natl Acad. Sci. USA 103, 3333–3338 (2006).
Spencer, L. A. et al. Human eosinophils constitutively express multiple TH1, TH2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 85, 117–123 (2009).
Neves, J. S., Radke, A. L. & Weller, P. F. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J. Allergy Clin. Immunol. 125, 477–482 (2010).
Neves, J. S. et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc. Natl Acad. Sci. USA 105, 18478–18483 (2008).
Wright, B. L., Leiferman, K. M. & Gleich, G. J. Eosinophil granule protein localization in eosinophilic endomyocardial disease. N. Engl. J. Med. 365, 187–188 (2011).
Drage, L. A. et al. Evidence for pathogenic involvement of eosinophils and neutrophils in Churg–Strauss syndrome. J. Am. Acad. Dermatol. 47, 209–216 (2002).
Saffari, H. et al. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2013.11.024 (2014).
Blanchard, C. & Rothenberg, M. E. Biology of the eosinophil. Adv. Immunol. 101, 81–121 (2009).
Rosenberg, H. F., Dyer, K. D. & Foster, P. S. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22 (2013).
Lee, J. J., Jacobsen, E. A., McGarry, M. P., Schleimer, R. P. & Lee, N. A. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy 40, 563–575 (2010).
Chu, V. T. et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12, 151–159 (2011).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Noguchi, H., Kephart, G. M., Colby, T. V. & Gleich, G. J. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am. J. Pathol. 140, 521–528 (1992).
Gundel, R. H., Letts, L. G. & Gleich, G. J. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J. Clin. Invest. 87, 1470–1473 (1991).
Fryer, A. D., Adamko, D. J., Yost, B. L. & Jacoby, D. B. Effects of inflammatory cells on neuronal M2 muscarinic receptor function in the lung. Life Sci. 64, 449–455 (1999).
Bousquet, J. et al. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J. Allergy Clin. Immunol. 88, 649–660 (1991).
Filley, W. V., Holley, K. E., Kephart, G. M. & Gleich, G. J. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet 2, 11–16 (1982).
Kephart, G. M. et al. Marked deposition of eosinophil-derived neurotoxin in adult patients with eosinophilic esophagitis. Am. J. Gastroenterol. 105, 298–307 (2010).
Levi-Schaffer, F. et al. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-β). Proc. Natl Acad. Sci. USA 96, 9660–9665 (1999).
Humbles, A. A. et al. A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 (2004).
Zagai, U., Skold, C. M., Trulson, A., Venge, P. & Lundahl, J. The effect of eosinophils on collagen gel contraction and implications for tissue remodelling. Clin. Exp. Immunol. 135, 427–433 (2004).
Gomes, I. et al. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J. Allergy Clin. Immunol. 116, 796–804 (2005).
Zagai, U., Dadfar, E., Lundahl, J., Venge, P. & Skold, C. M. Eosinophil cationic protein stimulates TGF-β1 release by human lung fibroblasts in vitro. Inflammation 30, 153–160 (2007).
Ames, P. R., Margaglione, M., Mackie, S. & Alves, J. D. Eosinophilia and thrombophilia in Churg–Strauss syndrome: a clinical and pathogenetic overview. Clin. Appl. Thromb. Hemost. 16, 628–636 (2010).
Moosbauer, C. et al. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood 109, 995–1002 (2007).
Mukai, H. Y., Ninomiya, H., Ohtani, K., Nagasawa, T. & Abe, T. Major basic protein binding to thrombomodulin potentially contributes to the thrombosis in patients with eosinophilia. Br. J. Haematol. 90, 892–899 (1995).
Slungaard, A., Vercellotti, G. M., Tran, T., Gleich, G. J. & Key, N. S. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J. Clin. Invest. 91, 1721–1730 (1993).
Rohrbach, M. S., Wheatley, C. L., Slifman, N. R. & Gleich, G. J. Activation of platelets by eosinophil granule proteins. J. Exp. Med. 172, 1271–1274 (1990).
Dorfman, L. J., Ransom, B. R., Forno, L. S. & Kelts, A. Neuropathy in the hypereosinophilic syndrome. Muscle Nerve 6, 291–298 (1983).
Wichman, A., Buchthal, F., Pezeshkpour, G. H. & Fauci, A. S. Peripheral neuropathy in hypereosinophilic syndrome. Neurology 35, 1140–1145 (1985).
Nascimento, O., De Freitas, M., Chimelli, L. & Scaravilli, F. Peripheral neuropathy in hypereosinophilic syndrome with vasculitis. Arq. Neuropsiquiatr. 49, 450–455 (1991).
Costello, R. W. et al. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am. J. Physiol. 273, L93–L103 (1997).
Kingham, P. J. et al. Effects of eosinophils on nerve cell morphology and development: the role of reactive oxygen species and p38 MAP kinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L915–L924 (2003).
Churg, J. & Strauss, L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am. J. Pathol. 27, 277–301 (1951).
Jennette, J. C. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Lanham, J. G., Elkon, K. B., Pusey, C. D. & Hughes, G. R. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg–Strauss syndrome. Medicine (Baltimore) 63, 65–81 (1984).
Comarmond, C. et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 65, 270–281 (2013).
Grayson, P. C. et al. New features of disease after diagnosis in 6 forms of systemic vasculitis. J. Rheumatol. 40, 1905–1912 (2013).
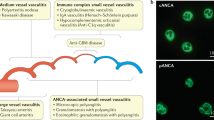
Sable-Fourtassou, R. et al. Antineutrophil cytoplasmic antibodies and the Churg–Strauss syndrome. Ann. Intern. Med. 143, 632–638 (2005).
Sinico, R. A. et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg–Strauss syndrome. Arthritis Rheum. 52, 2926–2935 (2005).
Sinico, R. A., Bottero, P. & Guillevin, L. Antineutrophil cytoplasmic autoantibodies and clinical phenotype in patients with Churg–Strauss syndrome. J. Allergy Clin. Immunol. 130, 1440 (2012).
Moosig, F. et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg–Strauss, EGPA): monocentric experiences in 150 patients. Ann. Rheum. Dis. 72, 1011–1017 (2013).
Khoury, P. et al. Serum biomarkers are similar in Churg–Strauss syndrome and hypereosinophilic syndrome. Allergy 67, 1149–1156 (2012).
Valent, P. et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev. Hematol. 5, 157–176 (2012).
Mahr, A. et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): evolutions in classification, etiopathogenesis, assessment and management. Curr. Opin. Rheumatol. 26, 16–23 (2014).
Vaglio, A. et al. HLA-DRB4 as a genetic risk factor for Churg–Strauss syndrome. Arthritis Rheum. 56, 3159–3166 (2007).
Wenzel, S. E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med. 18, 716–725 (2012).
Miranda, C., Busacker, A., Balzar, S., Trudeau, J. & Wenzel, S. E. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J. Allergy Clin. Immunol. 113, 101–108 (2004).
Nair, P. et al. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy 68, 1177–1184 (2013).
Lie, J. T. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum. 33, 1074–1087 (1990).
Katzenstein, A. L. Diagnostic features and differential diagnosis of Churg–Strauss syndrome in the lung. A review. Am. J. Clin. Pathol. 114, 767–772 (2000).
Churg, A. Recent advances in the diagnosis of Churg–Strauss syndrome. Mod. Pathol. 14, 1284–1293 (2001).
Xiao, H. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 (2002).
Salama, A. D. & Little, M. A. Animal models of antineutrophil cytoplasm antibody-associated vasculitis. Curr. Opin. Rheumatol. 24, 1–7 (2012).
Ishii, T. et al. Establishment of experimental eosinophilic vasculitis by IgE-mediated cutaneous reverse passive Arthus reaction. Am. J. Pathol. 174, 2225–2233 (2009).
Kiene, M. et al. Elevated interleukin-4 and interleukin-13 production by T cell lines from patients with Churg–Strauss syndrome. Arthritis Rheum. 44, 469–473 (2001).
Jakiela, B. et al. Increased production of IL-5 and dominant TH2-type response in airways of Churg–Strauss syndrome patients. Rheumatology (Oxford) 51, 1887–1893 (2012).
Muschen, M. et al. Involvement of soluble CD95 in Churg–Strauss syndrome. Am. J. Pathol. 155, 915–925 (1999).
Terrier, B. et al. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg–Strauss syndrome. Blood 116, 4523–4531 (2010).
Brusselle, G. G., Maes, T. & Bracke, K. R. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat. Med. 19, 977–979 (2013).
Jakiela, B. et al. Both TH2 and TH17 responses are involved in the pathogenesis of Churg–Strauss syndrome. Clin. Exp. Rheumatol. 29 (Suppl. 64), S23–S34 (2011).
Saito, H., Tsurikisawa, N., Tsuburai, T. & Akiyama, K. Involvement of regulatory T cells in the pathogenesis of Churg–Strauss syndrome. Int. Arch. Allergy Immunol. 146 (Suppl. 1), 73–76 (2008).
Tsurikisawa, N., Saito, H., Oshikata, C., Tsuburai, T. & Akiyama, K. Decreases in the numbers of peripheral blood regulatory T cells, and increases in the levels of memory and activated B cells, in patients with active eosinophilic granulomatosis and polyangiitis. J. Clin. Immunol. 33, 965–976 (2013).
Manger, B. J. et al. IgE-containing circulating immune complexes in Churg–Strauss vasculitis. Scand. J. Immunol. 21, 369–373 (1985).
Pepper, R. J. et al. Rituximab is effective in the treatment of refractory Churg–Strauss syndrome and is associated with diminished T-cell interleukin-5 production. Rheumatology (Oxford) 47, 1104–1105 (2008).
Tomasson, G., Grayson, P. C., Mahr, A. D., Lavalley, M. & Merkel, P. A. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology (Oxford) 51, 100–109 (2012).
Vaglio, A. et al. IgG4 immune response in Churg–Strauss syndrome. Ann. Rheum. Dis. 71, 390–393 (2012).
Grayson, P. C. et al. Clinical value of commonly-measured laboratory tests in eosinophilic granulomatosis with polyangiitis (Churg–Strauss) [abstract #756]. Arthritis Rheum. 65 (Suppl. 10) S319 (2013).
Monach, P. A. Biomarkers in vasculitis. Curr. Opin. Rheumatol. 26, 24–30 (2014).
Meziane, H., Maakel, M. L., Vachier, I., Bousquet, J. & Chanez, P. Sputum eosinophilia in Churg–Strauss syndrome. Respir. Med. 95, 799–801 (2001).
Cianchetti, S. et al. Are sputum ECP and eosinophils differently associated with clinical and functional findings of asthma? Clin. Exp. Allergy 44, 673–680 (2013).
Guilpain, P. et al. Serum eosinophil cationic protein: a marker of disease activity in Churg–Strauss syndrome. Ann. N. Y. Acad. Sci. 1107, 392–399 (2007).
Polzer, K. et al. Eotaxin-3 is involved in Churg–Strauss syndrome—a serum marker closely correlating with disease activity. Rheumatology (Oxford) 47, 804–808 (2008).
Zwerina, J. et al. Eotaxin-3 in Churg–Strauss syndrome: a clinical and immunogenetic study. Rheumatology (Oxford) 50, 1823–1827 (2011).
Dallos, T. et al. CCL17/thymus and activation-related chemokine in Churg–Strauss syndrome. Arthritis Rheum. 62, 3496–3503 (2010).
Schmitt, W. H. et al. Churg–Strauss syndrome: serum markers of lymphocyte activation and endothelial damage. Arthritis Rheum. 41, 445–452 (1998).
Hauser, T. et al. The leucotriene receptor antagonist montelukast and the risk of Churg–Strauss syndrome: a case-crossover study. Thorax 63, 677–682 (2008).
Higashi, N. et al. Clinical features of asthmatic patients with increased urinary leukotriene E4 excretion (hyperleukotrienuria): involvement of chronic hyperplastic rhinosinusitis with nasal polyposis. J. Allergy Clin. Immunol. 113, 277–283 (2004).
Szczeklik, W. et al. 12-hydroxy-eicosatetraenoic acid (12-HETE): a biomarker of Churg–Strauss syndrome. Clin. Exp. Allergy 42, 513–522 (2012).
Mukhtyar, C. et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann. Rheum. Dis. 68, 310–317 (2009).
Jayne, D. et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N. Engl. J. Med. 349, 36–44 (2003).
De Groot, K. et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 52, 2461–2469 (2005).
Stone, J. H. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 363, 221–232 (2010).
Fulkerson, P. C. & Rothenberg, M. E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 12, 117–129 (2013).
Guillevin, L. et al. Prognostic factors in polyarteritis nodosa and Churg–Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 75, 17–28 (1996).
Kikkawa, Y. et al. Interferon-α inhibits airway eosinophilia and hyperresponsiveness in an animal asthma model [corrected]. Asia Pac. Allergy 2, 256–263 (2012).
Metzler, C., Csernok, E., Gross, W. L. & Hellmich, B. Interferon-α for maintenance of remission in Churg–Strauss syndrome: a long-term observational study. Clin. Exp. Rheumatol. 28, 24–30 (2010).
Thiel, J., Hassler, F., Salzer, U., Voll, R. E. & Venhoff, N. Rituximab in the treatment of refractory or relapsing eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome). Arthritis Res. Ther. 15, R133 (2013).
Tsurikisawa, N. et al. Treatment of Churg–Strauss syndrome with high-dose intravenous immunoglobulin. Ann. Allergy Asthma Immunol. 92, 80–87 (2004).
Druilhe, A., Letuve, S. & Pretolani, M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis 8, 481–495 (2003).
Corren, J. Inhibition of interleukin-5 for the treatment of eosinophilic diseases. Discov. Med. 13, 305–312 (2012).
Kim, S., Marigowda, G., Oren, E., Israel, E. & Wechsler, M. E. Mepolizumab as a steroid-sparing treatment option in patients with Churg–Strauss syndrome. J. Allergy Clin. Immunol. 125, 1336–1343 (2010).
Herrmann, K., Gross, W. L. & Moosig, F. Extended follow-up after stopping mepolizumab in relapsing/refractory Churg–Strauss syndrome. Clin. Exp. Rheumatol. 30 (Suppl. 70), S62–S65 (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Balding, C. E., Howie, A. J., Drake-Lee, A. B. & Savage, C. O. TH2 dominance in nasal mucosa in patients with Wegener's granulomatosis. Clin. Exp. Immunol. 125, 332–339 (2001).
Choopong, P. et al. Eosinophil activation in Wegener's granulomatosis: a harbinger of disease progression? Ocul. Immunol. Inflamm. 13, 439–445 (2005).
Csernok, E. et al. Cytokine profiles in Wegener's granulomatosis: predominance of type 1 (TH1) in the granulomatous inflammation. Arthritis Rheum. 42, 742–750 (1999).
Krupsky, M., Landau, Z., Lifschitz-Mercer, B. & Resnitzky, P. Wegener's granulomatosis with peripheral eosinophilia. Atypical variant of a classic disease. Chest 104, 1290–1292 (1993).
Potter, M. B., Fincher, R. K. & Finger, D. R. Eosinophilia in Wegener's granulomatosis. Chest 116, 1480–1483 (1999).
Schmitt, W. H., Linder, R., Reinhold-Keller, E. & Gross, W. L. Improved differentiation between Churg–Strauss syndrome and Wegener's granulomatosis by an artificial neural network. Arthritis Rheum. 44, 1887–1896 (2001).
Schnabel, A., Csernok, E., Braun, J. & Gross, W. L. Activation of neutrophils, eosinophils, and lymphocytes in the lower respiratory tract in Wegener's granulomatosis. Am. J. Respir. Crit. Care Med. 161, 399–405 (2000).
Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children [Japanese]. Arerugi 16, 178–222 (1967).
Terai, M. et al. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatr. Infect. Dis. J. 21, 777–781 (2002).
Oner, T. et al. An observational study on peripheral blood eosinophilia in incomplete Kawasaki disease. Anadolu Kardiyol. Derg. 12, 160–164 (2012).
Bahrami, S., Malone, J. C., Webb, K. G. & Callen, J. P. Tissue eosinophilia as an indicator of drug-induced cutaneous small-vessel vasculitis. Arch. Dermatol. 142, 155–161 (2006).
Abu-Ghazaleh, R. I. et al. Eosinophil granule proteins in peripheral blood granulocytes. J. Leukoc. Biol. 52, 611–618 (1992).
Savage, C. O. Pathogenesis of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Clin. Exp. Immunol. 164 (Suppl. 1), 23–26 (2011).
Acknowledgements
This work was supported in whole or in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. The authors thank C. R. Lee for assistance with the preparation of Figure 3.
Author information
Authors and Affiliations
Contributions
All authors researched data for article, made a substantial contribution to discussion of content, wrote the article and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Khoury, P., Grayson, P. & Klion, A. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol 10, 474–483 (2014). https://doi.org/10.1038/nrrheum.2014.98
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.98
This article is cited by
-
Stellate ganglion block as an intervention in refractory eosinophilic granulomatosis with polyangiitis: a case report
Allergy, Asthma & Clinical Immunology (2022)
-
Intractable middle ear effusion in EGPA patients might cause permanent hearing loss: a case–control study
Allergy, Asthma & Clinical Immunology (2022)
-
Update Ätiopathogenese der Kleingefäßvaskulitis
Zeitschrift für Rheumatologie (2022)
-
Granulomatöse Vaskulitiden und Vaskulitiden mit extravaskulärer Granulomatose
Zeitschrift für Rheumatologie (2022)
-
Eosinophils in skin diseases
Seminars in Immunopathology (2021)