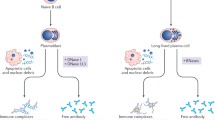
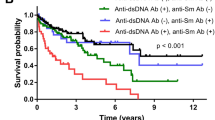
Key Points
-
Anti-double-stranded-DNA (dsDNA) antibodies are regarded as being central to the classification and pathogenesis of systemic lupus erythematosus
-
The nature of anti-dsDNA antibodies in clinical medicine is poorly defined; these antibodies can bind a spectrum of DNA and non-DNA structures
-
Anti-dsDNA antibodies are induced by a variety of different nucleic acids and non-DNA structures, which also determine whether the immune response is transient or sustained
-
Anti-dsDNA antibodies are pathogenic owing to their interaction with exposed chromatin, although pathogenicity by cross-reactivity with intrinsic renal structures cannot be excluded
-
Studies of the origin and impact of anti-dsDNA antibodies have provided insights into general aspects of the immune system and its control of immune tolerance
Abstract
The inclusion of 'the anti-DNA antibody' by the ACR and the Systemic Lupus International Collaborating Clinics (SLICC) as a criterion for systemic lupus erythematosus does not convey the diverse origins of these antibodies, whether their production is transient or persistent (which is heavily influenced by the nature of the inducing antigens), the specificities exerted by these antibodies or their clinical impact—or lack thereof. A substantial amount of data not considered in clinical medicine could be added from basic immunology evidence, which could change the paradigms linked to what 'the anti-DNA antibody' is, in a pathogenic, classification or diagnostic context.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Menzel, A. E. O. & Heidelberger, M. Cell protein fractions of bovine and avian tubercle bacillus strains and of the timothy-grass bacillus. J. Biol. Chem. 124, 301–307 (1938).
Sevag, M. G., Lackman, D. B. & Smolen, J. The isolation of the components of streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 124, 425–436 (1938).
Winkenwerder, W. L., Buell, M. V. & Howard, J. E. The sensitizing properties of the nucleic acids and their derivatives. Science 90, 356 (1939).
Ceppellini, R., Polli, E. & Celada, F. A DNA-reacting factor in serum of a patient with lupus erythematosus diffusus. Proc. Soc. Exp. Biol. Med. 96, 572–574 (1957).
Robbins, W. C., Holman, H. R., Deicher, H. & Kunkel, H. G. Complement fixation with cell nuclei and DNA in lupus erythematosus. Proc. Soc. Exp. Biol. Med. 96, 575–579 (1957).
Miescher, P. & Strassle, R. New serological methods for the detection of the L. E. factor. Vox Sang. 2, 283–287 (1957).
Seligman, M. Serology-evidence in serum from patients with disseminated lupus erythermatosus of a substance determining a precipitation reac tion with desoxyribonucleic acid [French]. C. R. Hebd. Seances Acad. Sci. 245, 243–245 (1957).
Stollar, B. D. Immunochemistry of DNA. Int. Rev. Immunol. 5, 1–22 (1989).
Stollar, B. D. Antibodies to DNA. CRC Crit. Rev. Biochem. 20, 1–36 (1986).
Madaio, M. P., Hodder, S., Schwartz, R. S. & Stollar, B. D. Responsiveness of autoimmune and normal mice to nucleic acid antigens. J. Immunol. 132, 872–876 (1984).
Pisetsky, D. S. & Vrabie, I. A. Antibodies to DNA: infection or genetics? Lupus 18, 1176–1180 (2009).
Lafer, E. M. et al. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J. Exp. Med. 153, 897–909 (1981).
Mostoslavsky, G. et al. Lupus anti-DNA autoantibodies crossreact with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur. J. Immunol. 31, 1221–1227 (2001).
Putterman, C. & Diamond, B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J. Exp. Med. 188, 29–38 (1998).
DeGiorgio, L. A. et al. A subset of lupus anti-DNA antibodies crossreacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001).
Stollar, B. D. Why the difference between B-DNA and Z-DNA? Lupus 6, 327–328 (1997).
Shlomchik, M. et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J. Exp. Med. 171, 265–292 (1990).
Marion, T. N., Krishnan, M. R., Steeves, M. A. & Desai, D. D. Affinity maturation and autoimmunity to DNA. Curr. Dir. Autoimmun. 6, 123–153 (2003).
Radic, M. Z. & Weigert, M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu. Rev. Immunol. 12, 487–520 (1994).
Desai, D. D., Krishnan, M. R., Swindle, J. T. & Marion, T. N. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. J. Immunol. 151, 1614–1626 (1993).
Rekvig, O. P., Bendiksen, S. & Moens, U. Immunity and autoimmunity induced by polyomaviruses: clinical, experimental and theoretical aspects. Adv. Exp. Med. Biol. 577, 117–147 (2006).
Rekvig, O. P. Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for SLE: critical remarks. Clin. Exp. Immunol. 179, 5–10 (2014).
Compagno, M. et al. Low diagnostic and predictive value of anti-dsDNA antibodies in unselected patients with recent onset of rheumatic symptoms: results from a long-term follow-up Scandinavian multicentre study. Scand. J. Rheumatol. 42, 311–316 (2013).
Krishnan, M. R., Wang, C. & Marion, T. N. Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kidney Int. 82, 184–192 (2012).
Seredkina, N., van der Vlag, J., Berden, J., Mortensen, E. & Rekvig, O. P. Lupus nephritis: enigmas, conflicting models and an emerging concept. Mol. Med. 19, 161–169 (2013).
Pisetsky, D. S. The role of innate immunity in the induction of autoimmunity. Autoimmun. Rev. 8, 69–72 (2008).
Krieg, A. M. & Vollmer, J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol. Rev. 220, 251–269 (2007).
Medzhitov, R. & Janeway, C. A. Jr. Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 (2002).
Christensen, S. R. & Shlomchik, M. J. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin. Immunol. 19, 11–23 (2007).
Schwartz, R. H. T cell clonal anergy. Curr. Opin. Immunol. 9, 351–357 (1997).
Foster, M. H. T cells and B cells in lupus nephritis. Semin. Nephrol. 27, 47–58 (2007).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Sandel, P. C. & Monroe, J. G. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity 10, 289–299 (1999).
Ehrlich, P. &, Morgenroth, J. Ueber Haemolysine: dritte Mittheilung [German]. Berlin Klin. Wochenschr. 37, 453–458 (1900).
Ehrlich, P. Ueber Hämolysine: fünfte Mittheilung [German]. Berlin Klin. Wochenschr. 38, 251–257 (1901).
Silverstein, A. M. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat. Immunol. 2, 279–281 (2001).
Blix, U., Iland, C. N. & Stacey, M. The serological activity of desoxypentosenucleic acids. Br. J. Exp. Pathol. 35, 241–251 (1954).
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995).
Yamamoto, S. et al. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN [correction of INF] and augment IFN-mediated [correction of INF] natural killer activity. J. Immunol. 148, 4072–4076 (1992).
Klinman, D. M., Yi, A. K., Beaucage, S. L., Conover, J. & Krieg, A. M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl Acad. Sci. USA 93, 2879–2883 (1996).
Sato, Y. et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science 273, 352–354 (1996).
Rock, K. L., Benacerraf, B. & Abbas, A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J. Exp. Med. 160, 1102–1113 (1984).
Sundar, K. et al. Expression of the Epstein-Barr virus nuclear antigen-1 (EBNA-1) in the mouse can elicit the production of anti-dsDNA and anti-Sm antibodies. J. Autoimmun. 23, 127–140 (2004).
Cerutti, M. L., Zarebski, L. M., de Prat, G. G. & Goldbaum, F. A. A viral DNA-binding domain elicits anti-DNA antibodies of different specificities. Mol. Immunol. 42, 327–333 (2005).
Moens, U. et al. In vivo expression of a single viral DNA-binding protein generates systemic lupus erythematosus-related autoimmunity to double-stranded DNA and histones. Proc. Natl Acad. Sci. USA 92, 12393–12397 (1995).
Van Ghelue, M., Moens, U., Bendiksen, S. & Rekvig, O. P. Autoimmunity to nucleosomes related to viral infection: a focus on hapten-carrier complex formation. J. Autoimmun. 20, 171–182 (2003).
Edgington, S. M. & Stollar, B. D. Immunogenicity of Z-DNA depends on the size of polynucleotide presented in complexes with methylated BSA. Mol. Immunol. 29, 609–617 (1992).
Rekvig, O. P. & Nossent, J. C. Anti-double-stranded DNA antibodies, nucleosomes, and systemic lupus erythematosus: a time for new paradigms? Arthritis Rheum. 48, 300–312 (2003).
Biermann, M. H. et al. The role of dead cell clearance in the aetiology and pathogenesis of systemic lupus erythematosus: dendritic cells as potential targets. Expert. Rev. Clin. Immunol. 10, 1151–1164 (2014).
Fenton, K. The effect of cell death in the initiation of lupus nephritis. Clin. Exp. Immunol. 179, 11–16 (2015).
Schroeder, K., Herrmann, M. & Winkler, T. H. The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity 46, 121–127 (2013).
Ghosh, A. & Bansal, M. A glossary of DNA structures from A to Z. Acta Crystallogr. D. Biol. Crystallogr. 59, 620–626 (2003).
Ha, S. C., Lowenhaupt, K., Rich, A., Kim, Y. G. & Kim, K. K. Crystal structure of a junction between B-DNA and Z-DNA reveals two extruded bases. Nature 437, 1183–1186 (2005).
Rothenburg, S., Koch-Nolte, F. & Haag, F. DNA methylation and Z.-DNA formation as mediators of quantitative differences in the expression of alleles. Immunol. Rev. 184, 286–298 (2001).
Rekvig, O. P. et al. Experimental expression in mice and spontaneous expression in human SLE of polyomavirus T-antigen. A molecular basis for induction of antibodies to DNA and eukaryotic transcription factors. J. Clin. I nvest. 99, 2045–2054 (1997).
Hahn, B. H. Antibodies to DNA. N. Engl. J. Med. 338, 1359–1368 (1998).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25, 1271–1277 (1982).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Widom, J. A relationship between the helical twist of DNA and the ordered positioning of nucleosomes in all eukaryotic cells. Proc. Natl Acad. Sci. USA 89, 1095–1099 (1992).
Richmond, T. J. & Davey, C. A. The structure of DNA in the nucleosome core. Nature 423, 145–150 (2003).
Griffith, J., Bleyman, M., Rauch, C. A., Kitchin, P. A. & Englund, P. T. Visualization of the bent helix in kinetoplast DNA by electron microscopy. Cell 46, 717–724 (1986).
Stollar, B. D. The experimental induction of antibodies to nucleic acids. Methods Enzymol. 70, 70–85 (1980).
Isenberg, D. A., Manson, J. J., Ehrenstein, M. R. & Rahman, A. Fifty years of anti-dsDNA antibodies: are we approaching journey's end? Rheumatology (Oxford) 46, 1052–1056 (2007).
Amital, H. et al. Treatment with a laminin-derived peptide suppresses lupus nephritis. J. Immunol. 175, 5516–5523 (2005).
Wellmann, U. et al. The evolution of human anti-double-stranded DNA autoantibodies. Proc. Natl Acad. Sci. USA 102, 9258–9263 (2005).
Olins, A. L. & Olins, D. E. Spheroid chromatin units (v bodies). Science 183, 330–332 (1974).
Kornberg, R. D. & Lorch, Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98, 285–294 (1999).
Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251–260 (1997).
de Graaf, C. A. & van Stenseel, B. Chromatin organization: form to function. Curr. Opin. Genet. Dev. 23, 185–190 (2013).
van Steensel, B. Chromatin: constructing the big picture. EMBO J. 30, 1885–1895 (2011).
Woodcock, C. L. & Ghosh, R. P. Chromatin higher-order structure and dynamics. Cold Spring Harb. Perspect. Biol. 2, a000596 (2010).
Haugbro, K., Nossent, J. C., Winkler, T., Figenschau, Y. & Rekvig, O. P. Anti-dsDNA antibodies and disease classification in antinuclear antibody positive patients: the role of analytical diversity. Ann. Rheum. Dis. 63, 386–394 (2004).
Compagno, M. et al. Clinical phenotype associations with various types of anti-dsDNA antibodies in patients with recent onset of rheumatic symptoms. Results from a multicentre observational study. Lupus Sci. Med. 1, e000007 (2014).
Neogi, T., Gladman, D. D., Ibanez, D. & Urowitz, M. Anti-dsDNA antibody testing by Farr and ELISA techniques is not equivalent. J. Rheumatol. 33, 1785–1788 (2006).
Rekvig, O. P., van der Vlag, J. & Seredkina, N. Anti-nucleosome antibodies—a critical reflection on their specificities and diagnostic impact. Arthritis Rheumatol. 66, 1061–1069 (2014).
Marion, T. N. et al. Immunoglobulin variable-region structures in immunity and autoimmunity to DNA. Tohoku J. Exp. Med. 173, 43–63 (1994).
Williams, R. C. Jr, Malone, C. C., Meyers, C., Decker, P. & Muller, S. Detection of nucleosome particles in serum and plasma from patients with systemic lupus erythematosus using monoclonal antibody 4H7. J. Rheumatol. 28, 81–94 (2001).
Kramers, K. et al. Specificity of monoclonal anti-nucleosome auto-antibodies derived from lupus mice. J. Autoimmun. 9, 723–729 (1996).
Stemmer, C., Briand, J. P. & Muller, S. Mapping of linear histone regions exposed at the surface of the nucleosome in solution. J. Mol. Biol. 273, 52–60 (1997).
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell co-stimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Voll, R. E. et al. Histone-specific TH0 and TH1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. 40, 2162–2171 (1997).
Datta, S. K. Production of pathogenic antibodies: cognate interactions between autoimmune T and B cells. Lupus. 7, 591–596 (1998).
Rekvig, O. P. et al. Molecular analyses of anti-DNA antibodies induced by polyomavirus BK in BALB/c mice. Scand. J. Immunol. 41, 593–602 (1995).
Craft, J. E. & Hardin, J. A. Linked sets of antinuclear antibodies: what do they mean? J. Rheumatol. Suppl. 14 (Suppl. 13), 106–109 (1987).
Radic, M., Herrmann, M., van der Vlag, J. & Rekvig, O. P. Regulatory and pathogenetic mechanisms of autoantibodies in SLE. Autoimmunity 44, 349–356 (2011).
Munoz, L. E., Lauber, K., Schiller, M., Manfredi, A. A. & Herrmann, M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 6, 280–289 (2010).
Andreassen, K., Moens, U., Nossent, H., Marion, T. N. & Rekvig, O. P. Termination of human T cell tolerance to histones by presentation of histones and polyomavirus T-antigen provided that T-antigen is complexed with nucleosomes. Arthritis Rheum. 42, 2449–2460 (1999).
Andreassen, K. et al. T cell autoimmunity to histones and nucleosomes is a latent property of the normal immune system. Arthritis Rheum. 46, 1270–1281 (2002).
Jenkins, M. K. The role of cell division in the induction of clonal anergy. Immunol. Today 13, 69–73 (1992).
Dure, M. & Macian, F. IL-2 signalling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Mol. Immunol. 46, 999–1006 (2009).
Rekvig, O. P. & van der Vlag, J. . The pathogenesis and diagnosis of systemic lupus erythematosus: still not resolved. Semin. Immunopathol. 36, 301–311 (2014).
Smeenk, R. J. et al. Anti-dsDNA: choice of assay in relation to clinical value. Rheumatol. Int. 11, 101–107 (1991).
Winfield, J. B., Faiferman, I. & Koffler, D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J. Clin. Invest. 59, 90–96 (1977).
Smeenk, R. & Aarden, L. The use of polyethylene glycol precipitation to detect low-avidity anti-DNA antibodies in systemic lupus erythematosus. J. Immunol. Methods 39, 165–180 (1980).
Isenberg, D. A. Autoantibodies: markers of disease or pathogenic? Ann. N. Y. Acad. Sci. 823, 256–262 (1997).
Rose, N. R. & Bona, C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol. Today 14, 426–430 (1993).
Falkow, S. Molecular Koch's postulates applied to bacterial pathogenicity—a personal recollection 15 years later. Nat. Rev. Microbiol. 2, 67–72 (2004).
Izui, S., Lambert, P. H., Fournie, G. J., Turler, H. & Miescher, P. A. Features of systemic lupus erythematosus in mice injected with bacterial lipopolysaccharides: identificantion of circulating DNA and renal localization of DNA-anti-DNA complexes. J. Exp. Med. 145, 1115–1130 (1977).
Mjelle, J. E., Kalaaji, M. & Rekvig, O. P. Exposure of chromatin and not high affinity for dsDNA determines the nephritogenic impact of anti-dsDNA antibodies in (NZB×NZW)F1 mice. Autoimmunity 42, 104–111 (2009).
Xie, C., Liang, Z., Chang, S. & Mohan, C. Use of a novel elution regimen reveals the dominance of polyreactive antinuclear autoantibodies in lupus kidneys. Arthritis Rheum. 48, 2343–2352 (2003).
Van Bruggen, M. C., Kramers, C., Hylkema, M. N., Smeenk, R. J. & Berden, J. H. Significance of anti-nuclear and anti-extracellular matrix autoantibodies for albuminuria in murine lupus nephritis; a longitudinal study on plasma and glomerular eluates in MRL/l mice. Clin. Exp. Immunol. 105, 132–139 (1996).
Yung, S., Cheung, K. F., Zhang, Q. & Chan, T. M. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J. Am. Soc. Nephrol. 21, 1912–1927 (2010).
Sun, K. H. et al. Anti-dsDNA autoantibody crossreacts with the C-terminal hydrophobic cluster region containing phenylalanines in the acidic ribosomal phosphoprotein P1 to exert a cytostatic effect on the cells. Biochem. Biophys. Res. Commun. 263, 334–339 (1999).
Mjelle, J. E., Rekvig, O. P. & Fenton, K. A. Nucleosomes possess a high affinity for glomerular laminin and collagen IV and bind nephritogenic antibodies in murine lupus-like nephritis. Ann. Rheum. Dis. 66, 1661–1668 (2007).
Ehrenstein, M. R. et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 48, 705–711 (1995).
Fenton, K. A., Tommeras, B., Marion, T. N. & Rekvig, O. P. Pure anti-dsDNA mAbs need chromatin structures to promote glomerular mesangial deposits in BALB/c mice. Autoimmunity 43, 179–188 (2009).
Adu, D., Dobson, J. & Williams, D. G. DNA-anti-DNA circulating complexes in the nephritis of systemic lupus erythematosus. Clin. Exp. Immunol. 43, 605–614 (1981).
Eilat, D. Crossreactions of anti-DNA antibodies and the central dogma of lupus nephritis. Immunol. Today 6, 123–127 (1985).
Fenton, K. et al. Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZB×NZW)F1 mice. PLoS ONE 4, e8474 (2009).
Kalaaji, M., Sturfelt, G., Mjelle, J. E., Nossent, H. & Rekvig, O. P. Critical comparative analyses of anti-α-actinin and glomerulus-bound antibodies in human and murine lupus nephritis. Arthritis Rheum. 54, 914–926 (2006).
Kalaaji, M., Mortensen, E., Jorgensen, L., Olsen, R. & Rekvig, O. P. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am. J. Pathol. 168, 1779–1792 (2006).
Kalaaji, M. et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 71, 664–672 (2007).
Seredkina, N., Zykova, S. N. & Rekvig, O. P. Progression of murine lupus nephritis is linked to acquired renal Dnase1 deficiency and not to upregulated apoptosis. Am. J. Pathol. 175, 97–106 (2009).
van der Vlag, J. & Berden, J. H. Lupus nephritis: role of antinucleosome autoantibodies. Semin. Nephrol. 31, 376–389 (2011).
LeBlanc, B. A., Urowitz, M. B. & Gladman, O. D. Serologically active, clinically quiescent systemic lupus erythematosus—longterm followup. J. Rheumatol. 21, 174–175 (1994).
Gladman, D. D., Urowitz, M. B. & Keystone, E. C. Serologically active clinically quiescent systemic lupus erythematosus: a discordance between clinical and serologic features. Am. J. Med. 66, 210–215 (1979).
Grootscholten, C. et al. Deposition of nucleosomal antigens (histones and DNA) in the epidermal basement membrane in human lupus nephritis. Arthritis Rheum. 48, 1355–1362 (2003).
Hedberg, A., Fismen, S., Fenton, K. A., Mortensen, E. S. & Rekvig, O. P. Deposition of chromatin-IgG complexes in skin of nephritic MRL-lpr/lpr mice is associated with increased local matrix metalloprotease activities. Exp. Dermatol. 19, e265–e274 (2010).
Fismen, S. et al. Circulating chromatin-anti-chromatin antibody complexes bind with high affinity to dermo-epidermal structures in murine and human lupus nephritis. Lupus 18, 597–607 (2009).
Huerta, P. T., Kowal, C., DeGiorgio, L. A., Volpe, B. T. & Diamond, B. Immunity and behaviour: antibodies alter emotion. Proc. Natl Acad. Sci. USA 103, 678–683 (2006).
Rajan, T. V. The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 24, 376–379 (2003).
Krishnan, M. R., Jou, N. T. & Marion, T. N. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J. Immunol. 157, 2430–2439 (1996).
Acknowledgements
The author thanks Rod Wolstenholme, Section for Dissemination Services, for expert help in preparing Figures 1,2,3,4,5, and Hege Lynum Pedersen and Professor Bjarne Østerud, both from the Department of Medical Biology, Faculty of Health Sciences, Tromsø, Norway, for critical reading of the manuscript and for helpful suggestions. This study was supported by the University of Tromsø as Milieu Support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Rekvig, O. The anti-DNA antibody: origin and impact, dogmas and controversies. Nat Rev Rheumatol 11, 530–540 (2015). https://doi.org/10.1038/nrrheum.2015.69
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.69
This article is cited by
-
Diagnostic role of anti-dsDNA antibodies: do not forget autoimmune hepatitis
Nature Reviews Rheumatology (2021)
-
New insights into the role of antinuclear antibodies in systemic lupus erythematosus
Nature Reviews Rheumatology (2020)
-
Anti-Smith antibody is associated with disease activity in patients with new-onset systemic lupus erythematosus
Rheumatology International (2019)
-
Anti-DNA antibodies — quintessential biomarkers of SLE
Nature Reviews Rheumatology (2016)
-
Update on Biologic Therapies for Systemic Lupus Erythematosus
Current Rheumatology Reports (2016)