Key Points
-
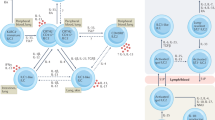
Dendritic cells (DCs) have critical roles in autoimmune disorders and functional heterogeneity exist within each DC subset
-
Autoimmune diseases are associated with changes in DC distribution and function
-
Some DC functions are similarly altered in different autoimmune diseases, whereas other changes are more disease-specific
-
DCs can be manipulated to restore T-cell tolerance and modulate autoantibody production
Abstract
Dendritic cells (DCs) are central regulators of the balance between immunity and tolerance, and alteration of the specialized DC system is a common feature of both systemic and tissue-specific autoimmune diseases. Increasing evidence indicates that the heterogeneity and the remarkable functional diversity of DC subsets might be differentially affected in autoimmune disorders, which accounts for different pathologies. This Review discusses recent findings that support this concept and provides a new conceptual overview of the altered function and distribution of DCs in autoimmune disorders. The discussion will focus on systemic lupus erythematosus — a prototype of a multi-organ disease — as well as rheumatoid arthritis and idiopathic inflammatory myopathies, pathologies characterized by tissue-specific lesions. Studies on these diseases have revealed common and disease-specific changes in DC distribution and in critical DC functions, such as phagocytosis, cytokine secretion and migration. An improved understanding of the roles of altered DC distribution and/or disturbed key functions in these autoimmune diseases will pave the way for the development of new therapies aiming at reducing immunogenicity and at enhancing the tolerogenic capacity of DCs. Although some tolerogenic DCs have already been introduced in the clinic, the successful translation of other DC-based therapies will require considerable research efforts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dalakas, M. C. Inflammatory muscle diseases. N. Engl. J. Med. 372, 1734–1747 (2015).
Hahn, B. H. Antibodies to DNA. N. Engl. J. Med. 338, 1359–1368 (1998).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Koenig, M., Fritzler, M. J., Targoff, I. N., Troyanov, Y. & Senécal, J.-L. Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights into clinical features and outcomes. Arthritis Res. Ther. 9, R78 (2007).
Tsokos, G. C. Systemic lupus erythematosus in 2015: Cellular and metabolic requirements of effector T cells. Nat. Rev. Rheumatol. 12, 74–76 (2016).
Petrelli, A. & van Wijk, F. CD8+ T cells in human autoimmune arthritis: the unusual suspects. Nat. Rev. Rheumatol. 12, 421–428 (2016).
Malmström, V., Venalis, P. & Albrecht, I. T cells in myositis. Arthritis Res. Ther. 14, 230 (2012).
Pascual, V., Farkas, L. & Banchereau, J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr. Opin. Immunol. 18, 676–682 (2006).
Miossec, P. & Kolls, J. K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 11, 763–776 (2012).
Goebels, N. et al. Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermatomyositis. J. Clin. Invest. 97, 2905–2910 (1996).
Zielinski, C. E. Autoimmunity beyond TH17: GM-CSF producing T cells. Cell Cycle 13, 2489–2490 (2014).
Ganguly, D., Haak, S., Sisirak, V. & Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 13, 566–577 (2013).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578 (2014).
Siegal, F. P. et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 (1999).
León, B., López-Bravo, M. & Ardavín, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 (2007).
Hammad, H. et al. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of TH2 immunity to inhaled house dust mite allergen. J. Exp. Med. 207, 2097–2111 (2010).
Campbell, I. K. et al. Differentiation of inflammatory dendritic cells is mediated by NF-κB1-dependent GM-CSF production in CD4 T cells. J. Immunol. Baltim. Md. 186, 5468–5477 (2011).
Segura, E. et al. Human inflammatory dendritic cells induce TH17 cell differentiation. Immunity 38, 336–348 (2013).
Steinman, R. M., Inaba, K., Turley, S., Pierre, P. & Mellman, I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum. Immunol. 60, 562–567 (1999).
Somersan, S. & Bhardwaj, N. Tethering and tickling: a new role for the phosphatidylserine receptor. J. Cell Biol. 155, 501–504 (2001).
Steinman, R. M. et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann. NY Acad. Sci. 987, 15–25 (2003).
Janeway, C. A. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Gallucci, S., Lolkema, M. & Matzinger, P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5, 1249–1255 (1999).
Gallo, P. M. & Gallucci, S. The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity. Front. Immunol. 4, 138 (2013).
Walsh, K. P. & Mills, K. H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 34, 521–530 (2013).
Segura, E. et al. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 209, 653–660 (2012).
Schlitzer, A. et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 (2013).
Yu, C. I. et al. Human CD141+ dendritic cells induce CD4+ T cells to produce type 2 cytokines. J. Immunol. Baltim. Md. 1950193, 4335–4343 (2014).
Villadangos, J. A. & Young, L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 (2008).
Reizis, B., Colonna, M., Trinchieri, G., Barrat, F. & Gilliet, M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat. Rev. Immunol. 11, 558–565 (2011).
Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015).
Moseman, E. A. et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. Baltim. Md. 1950173, 4433–4442 (2004).
Hanabuchi, S. et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J. Immunol. Baltim. Md. 1950184, 2999–3007 (2010).
Martín-Gayo, E., Sierra-Filardi, E., Corbí, A. L. & Toribio, M. L. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood 115, 5366–5375 (2010).
Jongbloed, S. L. et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207, 1247–1260 (2010).
Nizzoli, G. et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 122, 932–942 (2013).
Hémont, C., Neel, A., Heslan, M., Braudeau, C. & Josien, R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. J. Leukoc. Biol. 93, 599–609 (2013).
Ueno, H., Schmitt, N., Palucka, A. K. & Banchereau, J. Dendritic cells and humoral immunity in humans. Immunol. Cell Biol. 88, 376–380 (2010).
Jego, G., Pascual, V., Palucka, A. K. & Banchereau, J. Dendritic cells control B cell growth and differentiation. Curr. Dir. Autoimmun. 8, 124–139 (2005).
Qi, H., Egen, J. G., Huang, A. Y. C. & Germain, R. N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 (2006).
MacLennan, I. & Vinuesa, C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity 17, 235–238 (2002).
Kalled, S. L., Ambrose, C. & Hsu, Y.-M. The biochemistry and biology of BAFF, APRIL and their receptors. Curr. Dir. Autoimmun. 8, 206–242 (2005).
Gill, M. A. et al. Blood dendritic cells and DC-poietins in systemic lupus erythematosus. Hum. Immunol. 63, 1172–1180 (2002).
Jin, O. et al. Systemic lupus erythematosus patients have increased number of circulating plasmacytoid dendritic cells, but decreased myeloid dendritic cells with deficient CD83 expression. Lupus 17, 654–662 (2008).
Cederblad, B. et al. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha- producing cells. J. Autoimmun. 11, 465–470 (1998).
Farkas, L., Beiske, K., Lund-Johansen, F., Brandtzaeg, P. & Jahnsen, F. L. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 (2001).
Migita, K. et al. Reduced blood BDCA-2+ (lymphoid) and CD11c+ (myeloid) dendritic cells in systemic lupus erythematosus. Clin. Exp. Immunol. 142, 84–91 (2005).
Jongbloed, S. L. et al. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res. Ther. 8, R15 (2006).
Page, G. & Miossec, P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J. Pathol. 204, 28–38 (2004).
Reis e Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 6, 476–483 (2006).
Sozzani, S., Vermi, W., Del Prete, A. & Facchetti, F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 31, 270–277 (2010).
Blomberg, S. et al. Presence of cutaneous interferon-α producing cells in patients with systemic lupus erythematosus. Lupus 10, 484–490 (2001).
Fiore, N. et al. Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol. Immunol. 45, 259–265 (2008).
Tucci, M. et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 58, 251–262 (2008).
Lebre, M. C. et al. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am. J. Pathol. 172, 940–950 (2008).
López de Padilla, C. M. et al. Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis Rheum. 56, 1658–1668 (2007).
Cella, M. et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5, 919–923 (1999).
Vermi, W. et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 201, 509–515 (2005).
Skrzeczyńska-Moncznik, J. et al. Potential role of chemerin in recruitment of plasmacytoid dendritic cells to diseased skin. Biochem. Biophys. Res. Commun. 380, 323–327 (2009).
Albanesi, C. et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 206, 249–258 (2009).
Kaneko, K. et al. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res. Ther. 13, R158 (2011).
Eisinger, K. et al. Chemerin induces CCL2 and TLR4 in synovial fibroblasts of patients with rheumatoid arthritis and osteoarthritis. Exp. Mol. Pathol. 92, 90–96 (2012).
Bondue, B., Wittamer, V. & Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 22, 331–338 (2011).
Eloranta, M.-L. et al. A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum. 56, 3112–3124 (2007).
Page, G., Chevrel, G. & Miossec, P. Anatomic localization of immature and mature dendritic cell subsets in dermatomyositis and polymyositis: Interaction with chemokines and Th1 cytokine-producing cells. Arthritis Rheum. 50, 199–208 (2004).
Liu, K. et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J. Exp. Med. 196, 1091–1097 (2002).
Morelli, A. E. et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood 101, 611–620 (2003).
Green, D. R., Ferguson, T., Zitvogel, L. & Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363 (2009).
Cohen, P. L. et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196, 135–140 (2002).
Leffler, J., Bengtsson, A. A. & Blom, A. M. The complement system in systemic lupus erythematosus: an update. Ann. Rheum. Dis. 73, 1601–1606 (2014).
Hu, C. Y. et al. Genetic polymorphism in milk fat globule-EGF factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus 18, 676–681 (2009).
Barrat, F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005).
Ramirez-Ortiz, Z. G. et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat. Immunol. 14, 917–926 (2013).
Asano, K. et al. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J. Exp. Med. 200, 459–467 (2004).
Peng, Y. & Elkon, K. B. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J. Clin. Invest. 121, 2221–2241 (2011).
White, S. & Rosen, A. Apoptosis in systemic lupus erythematosus. Curr. Opin. Rheumatol. 15, 557–562 (2003).
Baccala, R., Hoebe, K., Kono, D. H., Beutler, B. & Theofilopoulos, A. N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 13, 543–551 (2007).
Li, H. et al. Interferon-induced mechanosensing defects impede apoptotic cell clearance in lupus. J. Clin. Invest. 125, 2877–2890 (2015).
Tournadre, A., Lenief, V., Eljaafari, A. & Miossec, P. Immature muscle precursors are a source of interferon-β in myositis: role of Toll-like receptor 3 activation and contribution to HLA class I up-regulation. Arthritis Rheum. 64, 533–541 (2012).
Greter, M. et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity 36, 1031–1046 (2012).
Reynolds, G. et al. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-206578 (2015).
Benedetti, G. & Miossec, P. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur. J. Immunol. 44, 339–347 (2014).
Chevrel, G., Granet, C. & Miossec, P. Contribution of tumour necrosis factor α and interleukin (IL) 1β to IL6 production, NF-κB nuclear translocation, and class I MHC expression in muscle cells: in vitro regulation with specific cytokine inhibitors. Ann. Rheum. Dis. 64, 1257–1262 (2005).
Chevrel, G. et al. Interleukin-17 increases the effects of IL-1β on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. J. Neuroimmunol. 137, 125–133 (2003).
Benderdour, M. et al. Interleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1beta. J. Rheumatol. 29, 1262–1272 (2002).
Pacquelet, S. et al. Interleukin 17, a nitric oxide-producing cytokine with a peroxynitrite-independent inhibitory effect on proteoglycan synthesis. J. Rheumatol. 29, 2602–2610 (2002).
Takayanagi, H. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 5, 667–676 (2009).
Hot, A. & Miossec, P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann. Rheum. Dis. 70, 727–732 (2011).
Lee, S.-Y. et al. IL-17-mediated Bcl-2 expression regulates survival of fibroblast-like synoviocytes in rheumatoid arthritis through STAT3 activation. Arthritis Res. Ther. 15, R31 (2013).
Page, G. & Miossec, P. RANK and RANKL expression as markers of dendritic cell-T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis Rheum. 52, 2307–2312 (2005).
Takayanagi, H. et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605 (2000).
Rivollier, A. et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 104, 4029–4037 (2004).
Gilliet, M., Cao, W. & Liu, Y.-J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 (2008).
Braun, D., Caramalho, I. & Demengeot, J. IFN-α/β enhances BCR-dependent B cell responses. Int. Immunol. 14, 411–419 (2002).
Le Bon, A. et al. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14, 461–470 (2001).
Le Bon, A. et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. Baltim. Md. 1950176, 2074–2078 (2006).
Means, T. K. et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115, 407–417 (2005).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra40 (2013).
Lombardi, V., Van Overtvelt, L., Horiot, S. & Moingeon, P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-γ, and IL-17A by naive CD4+ T cells. J. Immunol. Baltim. Md. 182, 3372–3379 (2009).
Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Zhang, R. et al. Interferon-alpha and interleukin-6 in SLE serum induce the differentiation and maturation of dendritic cells derived from CD34+ hematopoietic precursor cells. Cytokine 50, 195–203 (2010).
Litinskiy, M. B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
MartIn-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Wakim, L. M., Waithman, J., van Rooijen, N., Heath, W. R. & Carbone, F. R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Tucci, M., Ciavarella, S., Strippoli, S., Dammacco, F. & Silvestris, F. Oversecretion of cytokines and chemokines in lupus nephritis is regulated by intraparenchymal dendritic cells: a review. Ann. NY Acad. Sci. 1173, 449–457 (2009).
Page, G., Lebecque, S. & Miossec, P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J. Immunol. Baltim. Md. 168, 5333–5341 (2002).
Noort, A. R. et al. Tertiary lymphoid structures in rheumatoid arthritis: NF-κB-inducing kinase-positive endothelial cells as central players. Am. J. Pathol. 185, 1935–1943 (2015).
Aziz, K. E., McCluskey, P. J. & Wakefield, D. Characterisation of follicular dendritic cells in labial salivary glands of patients with primary Sjögren syndrome: comparison with tonsillar lymphoid follicles. Ann. Rheum. Dis. 56, 140–143 (1997).
Manzo, A., Bombardieri, M., Humby, F. & Pitzalis, C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol. Rev. 233, 267–285 (2010).
Pitzalis, C., Kelly, S. & Humby, F. New learnings on the pathophysiology of RA from synovial biopsies. Curr. Opin. Rheumatol. 25, 334–344 (2013).
Corsiero, E. et al. Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol. Lett. 145, 62–67 (2012).
Rangel-Moreno, J. et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat. Immunol. 12, 639–646 (2011).
Humby, F. et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 6, e1 (2009).
Kroot, E. J. et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 43, 1831–1835 (2000).
Thomas, R. Dendritic cells and the promise of antigen-specific therapy in rheumatoid arthritis. Arthritis Res. Ther. 15, 204 (2013).
Daniel, C., Ploegh, H. & von Boehmer, H. Antigen-specific induction of regulatory T cells in vivo and in vitro. Methods Mol. Biol. 707, 173–185 (2011).
Idoyaga, J. et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Invest. 123, 844–854 (2013).
Benham, H. et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci. Transl. Med. 7, 290ra87 (2015).
Hilkens, C. M. U. & Isaacs, J. D. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin. Exp. Immunol. 172, 148–157 (2013).
US National Library of Medicine. Autologous Tolerogenic Dendritic Cells for Rheumatoid Arthritis (AutoDECRA) ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT01352858 (2013).
Rowland, S. L. et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 211, 1977–1991 (2014).
Sisirak, V. et al. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 211, 1969–1976 (2014).
Merrill, J. T. et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann. Rheum. Dis. 70, 1905–1913 (2011).
Petri, M. et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 65, 1011–1021 (2013).
McBride, J. M. et al. Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum. 64, 3666–3676 (2012).
Lauwerys, B. R. et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 65, 447–456 (2013).
Haniffa, M., Collin, M. & Ginhoux, F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv. Immunol. 120, 1–49 (2013).
Segura, E. & Amigorena, S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 34, 440–445 (2013).
Lee, J. et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J. Exp. Med. 212, 385–399 (2015).
Breton, G. et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J. Exp. Med. 212, 401–413 (2015).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of content and wrote the manuscript. P.M. reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Coutant, F., Miossec, P. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol 12, 703–715 (2016). https://doi.org/10.1038/nrrheum.2016.147
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.147
This article is cited by
-
Signaling pathways involved in the biological functions of dendritic cells and their implications for disease treatment
Molecular Biomedicine (2023)
-
New insights into inflammatory osteoclast precursors as therapeutic targets for rheumatoid arthritis and periodontitis
Bone Research (2023)
-
Metformin induces tolerogenicity of dendritic cells by promoting metabolic reprogramming
Cellular and Molecular Life Sciences (2023)
-
Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis
Journal of Neuroinflammation (2022)
-
Immunosuppressive properties of human PD-1 + , PDL-1 + and CD80 + dendritic cells from lymph nodes aspirates of lung cancer patients
Cancer Immunology, Immunotherapy (2022)