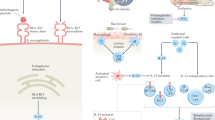
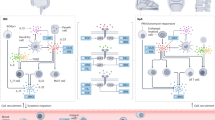
Key Points
-
Remission and low disease activity should be primary treatment targets for all patients with spondyloarthritis (SpA)
-
NSAIDs are the first-line treatment for SpA; TNF blockers are indicated for patients with axial SpA who do not respond to NSAIDs or in whom NSAIDs are contraindicated
-
Patients with nonradiographic axial SpA should have MRI evidence of inflammatory changes or elevated C-reactive protein levels to receive anti-TNF treatment
-
Despite the good anti-inflammatory activity of NSAIDs and TNF blockers, insufficient data exist about whether these drugs can prevent or retard progression of structural spinal damage in axial SpA
-
The IL-17 antagonist secukinumab is a new treatment option for patients with ankylosing spondylitis
-
Treatment options in peripheral SpA (except psoriatic arthritis) are currently limited to NSAIDs, DMARDs and steroids; biologics are not officially approved for this indication
Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) are the first-line treatment for patients with symptomatic axial spondyloarthritis (SpA), who seem to respond best if treated early in the course of disease. The effect of NSAIDs on radiographic disease progression is less clear. Conventional disease-modifying antirheumatic drugs (DMARDs) are not recommended in the treatment of axial SpA, either alone or in combination with TNF blockers. Patients with nonradiographic axial SpA (nr-axSpA) seem to respond as well to TNF blockers as do patients with radiographic axial SpA (ankylosing spondylitis (AS)). However, patients with nr-axSpA should, in addition to a high severity of symptoms, also have objective signs of inflammation, detectable by MRI or by C-reactive protein testing, to be treated with TNF blockers. Whether early TNF-blocker treatment can retard new bone formation needs clarification. In clinical trials, anti-IL-17 agents and TNF blockers showed similar efficacy in patients with AS. The potential of IL-23 blockade for treatment of axial SpA needs to be further investigated. Treatment options for peripheral SpA have been much less thoroughly investigated than those for axial SpA. However, some data indicate that TNF blockers are effective for treating peripheral arthritis, enthesitis and dactylitis, the typical manifestations of peripheral SpA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Rudwaleit, M. et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 70, 25–31 (2011).
Sieper, J. et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann. Rheum. Dis. 68, 784–788 (2009).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum. 27, 361–368 (1984).
Carter, J. D. et al. Chlamydiae as etiologic agents in chronic undifferentiated spondylarthritis. Arthritis Rheum. 60, 1311–1316 (2009).
Van Praet, L. et al. Degree of bone marrow oedema in sacroiliac joints of patients with axial spondyloarthritis is linked to gut inflammation and male sex: results from the GIANT cohort. Ann. Rheum. Dis. 73, 1186–1189 (2014).
Sieper, J. Developments in therapies for spondyloarthritis. Nat. Rev. Rheumatol. 8, 280–287 (2012).
Zochling, J. et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann. Rheum. Dis. 65, 442–452 (2006).
Braun, J. et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann. Rheum. Dis. 70, 896–904 (2011).
Ward, M. M. et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 68, 282–298 (2016).
van der Heijde, D. et al. 2010 update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann. Rheum. Dis. 70, 905–908 (2011).
Smolen, J. S. et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann. Rheum. Dis. 73, 6–16 (2014).
Dougados, M. et al. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 44, 180–185 (2001).
Dougados, M. et al. Ximoprofen in ankylosing spondylitis: a double blind placebo controlled dose ranging study. Scand. J. Rheumatol. 23, 243–248 (1994).
Sieper, J. et al. Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann. Rheum. Dis. 67, 323–329 (2008).
van der Heijde, D. et al. Evaluation of the efficacy of etoricoxib in ankylosing spondylitis: results of a fifty-two-week, randomized, controlled study. Arthritis Rheum. 52, 1205–1215 (2005).
Barkhuizen, A. et al. Celecoxib is efficacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J. Rheumatol. 33, 1805–1812 (2006).
Sieper, J. et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, Part 1. Ann. Rheum. Dis. 73, 101–107 (2014).
Anderson, J. J., Baron, G., van der Heijde, D., Felson, D. T. & Dougados, M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 44, 1876–1886 (2001).
Sieper, J. et al. Maintenance of biologic-free remission with naproxen or no treatment in patients with early, active axial spondyloarthritis: results from a 6-month, randomised, open-label follow-up study, INFAST Part 2. Ann. Rheum. Dis. 73, 108–113 (2014).
Kroon, F. P. et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst. Rev. 7, CD010952 (2015).
Wang, R., Dasgupta, A. & Ward, M. M. Comparative efficacy of non-steroidal anti-inflammatory drugs in ankylosing spondylitis: a Bayesian network meta-analysis of clinical trials. Ann. Rheum. Dis. (2015).
Coxib and traditional NSAID Trialists' (CNT) Collaboration et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382, 769–779 (2013).
Song, I. H., Poddubnyy, D. A., Rudwaleit, M. & Sieper, J. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 58, 929–938 (2008).
Bakland, G., Gran, J. T. & Nossent, J. C. Increased mortality in ankylosing spondylitis is related to disease activity. Ann. Rheum. Dis. 70, 1921–1925 (2011).
Haroon, N. N., Paterson, J. M., Li, P., Inman, R. D. & Haroon, N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann. Intern. Med. 163, 409–416 (2015).
Dougados, M. et al. Evaluation of the nonsteroidal anti-inflammatory drug-sparing effect of etanercept in axial spondyloarthritis: results of the multicenter, randomized, double-blind, placebo-controlled SPARSE study. Arthritis Res. Ther. 16, 481 (2014).
Molto, A. et al. Brief report: nonsteroidal antiinflammatory drug-sparing effect of tumor necrosis factor inhibitors in early axial spondyloarthritis: results from the DESIR cohort. Arthritis Rheumatol. 67, 2363–2368 (2015).
Haibel, H. et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann. Rheum. Dis. 66, 419–421 (2007).
Chen, J., Lin, S. & Liu, C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst. Rev. 11, CD004800 (2014).
Haibel, H., Rudwaleit, M., Braun, J. & Sieper, J. Six months open label trial of leflunomide in active ankylosing spondylitis. Ann. Rheum. Dis. 64, 124–126 (2005).
Braun, J. et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum. 63, 1543–1551 (2011).
Song, I. H. et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomised controlled trial. Ann. Rheum. Dis. 70, 590–596 (2011).
Braun, J. et al. Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicentre randomised controlled trial. Ann. Rheum. Dis. 65, 1147–1153 (2006).
Vincent, F. B. et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann. Rheum. Dis. 72, 165–178 (2013).
Breban, M. et al. Maintenance of infliximab treatment in ankylosing spondylitis: results of a one-year randomized controlled trial comparing systematic versus on-demand treatment. Arthritis Rheum. 58, 88–97 (2008).
Braun, J., Baraliakos, X., Kudrin, A., Kim, H. U. & Lee, S. J. Striking discrepancy in the development of anti-drug antibodies (ADA) in patients with rheumatoid arthritis (RA) and ankylosing spondylitis (AS) in response to infliximab (INF) and its biosimilar CT-P13. Presented at the 2014 ACR/ARHP Annual Meeting (2014).
Heiberg, M. S. et al. The comparative one-year performance of anti-tumor necrosis factor α drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 59, 234–240 (2008).
Lie, E. et al. The effect of comedication with conventional synthetic disease modifying antirheumatic drugs on TNF inhibitor drug survival in patients with ankylosing spondylitis and undifferentiated spondyloarthritis: results from a nationwide prospective study. Ann. Rheum. Dis. 74, 970–978 (2015).
Maksymowych, W. P. et al. A six-month randomized, controlled, double-blind, dose-response comparison of intravenous pamidronate (60 mg versus 10 mg) in the treatment of nonsteroidal antiinflammatory drug-refractory ankylosing spondylitis. Arthritis Rheum. 46, 766–773 (2002).
Haibel, H. et al. Efficacy of oral prednisolone in active ankylosing spondylitis: results of a double-blind, randomised, placebo-controlled short-term trial. Ann. Rheum. Dis. 73, 243–246 (2014).
Braun, J. et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 359, 1187–1193 (2002).
van der Heijde, D. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 52, 582–591 (2005).
Davis, J. C. et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 48, 3230–3236 (2003).
Davis, J. C. et al. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann. Rheum. Dis. 64, 1557–1562 (2005).
van der Heijde, D. et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 54, 2136–2146 (2006).
Inman, R. D. et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 58, 3402–3412 (2008).
Landewe, R. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann. Rheum. Dis. 73, 39–47 (2014).
Haibel, H. et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum. 58, 1981–1991 (2008).
Barkham, N. et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitis. Arthritis Rheum. 60, 946–954 (2009).
Sieper, J. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 72, 815–822 (2013).
Dougados, M. et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 66, 2091–2102 (2014).
Sieper, J. et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 67, 2702–2712 (2015).
Machado, P. et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann. Rheum. Dis. 70, 47–53 (2010).
Deodhar, A. et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration's comments and concerns. Arthritis Rheumatol. 66, 2649–2656 (2014).
Poddubnyy, D., Haibel, H., Braun, J., Rudwaleit, M. & Sieper, J. Brief report: clinical course over two years in patients with early nonradiographic axial spondyloarthritis and patients with ankylosing spondylitis not treated with tumor necrosis factor blockers: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheumatol. 67, 2369–2375 (2015).
Haibel, H. et al. Long-term efficacy of adalimumab after drug withdrawal and retreatment in patients with active non-radiographically evident axial spondyloarthritis who experience a flare. Arthritis Rheum. 65, 2211–2213 (2013).
Song, I. H. et al. Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann. Rheum. Dis. 71, 1212–1215 (2012).
US National Library of Science. ClinicalTrials.gov [online], (2016).
US National Library of Science. ClinicalTrials.gov [online], (2016).
US National Library of Science. ClinicalTrials.gov [online], (2016).
Sieper, J. et al. Effect of certolizumab pegol over ninety-six weeks in patients with axial spondyloarthritis: results from a phase III randomized trial. Arthritis Rheumatol. 67, 668–677 (2015).
Rudwaleit, M. et al. MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann. Rheum. Dis. 67, 1276–1281 (2008).
Molto, A., Paternotte, S., Claudepierre, P., Breban, M. & Dougados, M. Effectiveness of tumor necrosis factor α blockers in early axial spondyloarthritis: data from the DESIR cohort. Arthritis Rheumatol. 66, 1734–1744 (2014).
Ciurea, A. et al. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum. 65, 3096–3106 (2013).
Haibel, H., Rudwaleit, M., Listing, J. & Sieper, J. Open label trial of anakinra in active ankylosing spondylitis over 24 weeks. Ann. Rheum. Dis. 64, 296–298 (2005).
Song, I. H. et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann. Rheum. Dis. 70, 1108–1110 (2011).
Song, I. H. et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum. 62, 1290–1297 (2010).
Sieper, J., Porter-Brown, B., Thompson, L., Harari, O. & Dougados, M. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann. Rheum. Dis. 73, 95–100 (2014).
Sieper, J. et al. Sarilumab for the treatment of ankylosing spondylitis: results of a phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann. Rheum. Dis. 74, 1051–1057 (2015).
Nam, J. L. et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 73, 516–528 (2014).
Langley, R. G. et al. Secukinumab in plaque psoriasis — results of two phase 3 trials. N. Engl. J. Med. 371, 326–338 (2014).
Gordon, K. B. et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N. Engl. J. Med. 373, 136–144 (2015).
Papp, K. et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br. J. Dermatol. 173, 930–939 (2015).
Leonardi, C. L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
Papp, K. A. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371, 1675–1684 (2008).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382, 780–789 (2013).
Mease, P. J. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 373, 1329–1339 (2015).
Baeten, D. et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713 (2013).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
Sieper, J. et al. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a phase 3, randomized, placebo-controlled trial with subcutaneous loading and maintenance dosing. Arthritis Rheum. 66, S232 (2014).
Baeten, D. et al. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a 52-week phase 3 randomized placebo-controlled trial with intravenous loading and subcutaneous maintenance dosing. Arthritis Rheum. 66 (Suppl.), S360 (2014).
Poddubnyy, D., Hermann, K. G., Callhoff, J., Listing, J. & Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 73, 817–823 (2014).
US National Library of Science. ClinicalTrials.gov [online], (2016).
US National Library of Science. ClinicalTrials.gov [online], (2016).
US National Library of Science. ClinicalTrials.gov [online], (2016).
US National Library of Science. ClinicalTrials.gov [online], (2016).
van der Heijde, D. et al. Tofacitinib in patients with ankylosing spondylitis: a phase 2, 16-week, randomized, placebo-controlled, dose-ranging study [abstract]. Arthritis Rheumatol. 67 (Suppl. 10), 5L (2015).
Sandborn, W. J. et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N. Engl. J. Med. 367, 1519–1528 (2012).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Genovese, M. C. et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann. Rheum. Dis. 72, 863–869 (2013).
Smolen, J. et al. A phase 2 study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann. Rheum. Dis. 74 (Suppl. 2), 76 (2015).
Poddubnyy, D. et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann. Rheum. Dis. 70, 1369–1374 (2011).
Poddubnyy, D. et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum. 64, 1388–1398 (2012).
Ramiro, S. et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann. Rheum. Dis. 73, 1455–1461 (2014).
Sieper, J., Appel, H., Braun, J. & Rudwaleit, M. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum. 58, 649–656 (2008).
Boersma, J. W. Retardation of ossification of the lumbar vertebral column in ankylosing spondylitis by means of phenylbutazone. Scand. J. Rheumatol. 5, 60–64 (1976).
Wanders, A. et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 52, 1756–1765 (2005).
Kroon, F., Landewe, R., Dougados, M. & van der Heijde, D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann. Rheum. Dis. 71, 1623–1629 (2012).
Poddubnyy, D. et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann. Rheum. Dis. 71, 1616–1622 (2012).
Sieper, J. et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2015-207897 (2015).
van der Heijde, D. et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 58, 3063–3070 (2008).
van der Heijde, D. et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 58, 1324–1331 (2008).
van der Heijde, D. et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res. Ther. 11, R127 (2009).
Braun, J. et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann. Rheum. Dis. 73, 1107–1113 (2014).
Baraliakos, X., Haibel, H., Listing, J., Sieper, J. & Braun, J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann. Rheum. Dis. 73, 710–715 (2014).
Haroon, N. et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 65, 2645–2654 (2013).
Baraliakos, X. et al. Effect of interleukin-17A inhibition on spinal radiographic changes through 2 years in patients with active ankylosing spondylitis: results of a phase 3 study with secukinumab [abstract]. Arthritis Rheumatol. 67 (Suppl. 10), 6L (2015).
Gossec, L. et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2015-208337 (2015).
Coates, L. C. et al. Group for research and assessment of psoriasis and psoriatic arthritis: treatment recommendations for psoriatic arthritis 2015. Arthritis Rheumatol. http://dx.doi.org/10.1002/art.39573 (2016).
Dougados, M. et al. A randomised, multicentre, double-blind, placebo-controlled trial of etanercept in adults with refractory heel enthesitis in spondyloarthritis: the HEEL trial. Ann. Rheum. Dis. 69, 1430–1435 (2010).
Paramarta, J. E. et al. Efficacy and safety of adalimumab for the treatment of peripheral arthritis in spondyloarthritis patients without ankylosing spondylitis or psoriatic arthritis. Ann. Rheum. Dis. 72, 1793–1799 (2013).
Mease, P. et al. Randomized controlled trial of adalimumab in patients with nonpsoriatic peripheral spondyloarthritis. Arthritis Rheumatol. 67, 914–923 (2015).
Brown, M. A. et al. SAT0258 baseline MRI/CRP as predictors of response to etanercept in the management of patients with non-radiographic axial spondyloarthritis. Ann. Rheum. Dis. 74 (Suppl. 2), 752 (2015).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article and made substantial contributions to discussion of content, writing and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.S. declares that he has received honoraria, speaker fees and/or consultancy payments from Abbvie, Janssen, Eli Lilly, MSD/Merck, Novartis, Pfizer, Roche and UCB. D.P. declares that he has received honoraria, speaker fees and/or consultancy payments from Abbvie, Bristol Myers Squibb, Eli Lilly, Janssen, MSD/Merck, Novartis, Pfizer, Roche and UCB.
Rights and permissions
About this article
Cite this article
Sieper, J., Poddubnyy, D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol 12, 282–295 (2016). https://doi.org/10.1038/nrrheum.2016.42
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.42
This article is cited by
-
Programmed cell death 10 can be used as a potential biomarker for ankylosing spondylitis diagnosis and treatment
Spinal Cord (2024)
-
Transfer of microRNA-22-3p by M2 macrophage-derived extracellular vesicles facilitates the development of ankylosing spondylitis through the PER2-mediated Wnt/β-catenin axis
Cell Death Discovery (2022)
-
Expert recommendations on early diagnosis and referral of axial spondyloarthritis in the Kingdom of Saudi Arabia
Clinical Rheumatology (2022)
-
Machine Learning in Rheumatic Diseases
Clinical Reviews in Allergy & Immunology (2021)
-
Molecular mechanisms and clinical studies of iguratimod for the treatment of ankylosing spondylitis
Clinical Rheumatology (2021)