Key Points
-
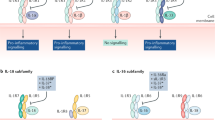
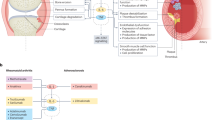
Toll-like receptors (TLRs) have an important role in chronic inflammation in rheumatic diseases, including rheumatoid arthritis, systemic lupus erythematosus, gout and Lyme disease
-
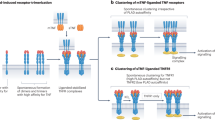
The Toll–IL-1 receptor (TIR) domain triggers biological responses to IL-1 molecules and to TLR ligands
-
Both microbial (such as lipopolysaccharide and DNA) and endogenous ligands have a role in chronic inflammation in rheumatic diseases
-
Novel strategies to inhibit TLR signalling to treat chronic inflammation in rheumatic diseases include antibodies and small molecules that target TLR receptors and TLR signalling
Abstract
In the past few years, new developments have been reported on the role of Toll-like receptors (TLRs) in chronic inflammation in rheumatic diseases. The inhibitory function of TLR10 has been demonstrated. Receptors that enhance the function of TLRs, and several TLR inhibitors, have been identified. In addition, the role of the microbiome and TLRs in the onset of rheumatic diseases has been reported. We review novel insights on the role of TLRs in several inflammatory joint diseases, including rheumatoid arthritis, systemic lupus erythematosus, gout and Lyme arthritis, with a focus on the signalling mechanisms mediated by the Toll–IL-1 receptor (TIR) domain, the exogenous and endogenous ligands of TLRs, and the current and future therapeutic strategies to target TLR signalling in rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Lemaitre, B. & Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 (2007).
Medzhitov, R. & Janeway, C. A. Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298 (1997).
Kimbrell, D. A. & Beutler, B. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2, 256–267 (2001).
Gao, D. et al. Cyclic GMP–AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906 (2013).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Anderson, K. V., Bokla, L. & Nusslein-Volhard, C. Establishment of dorsal–ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42, 791–798 (1985).
Nomura, N. et al. Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001–KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1. DNA Res. 1, 27–35 (1994).
Taguchi, T., Mitcham, J. L., Dower, S. K., Sims, J. E. & Testa, J. R. Chromosomal localization of TIL, a gene encoding a protein related to the Drosophila transmembrane receptor Toll, to human chromosome 4p14. Genomics 32, 486–488 (1996).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Takeda, K. & Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 (2005).
Oosting, M. et al. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc. Natl Acad. Sci. USA 111, E4478–E4484 (2014).
Roach, J. C. et al. The evolution of vertebrate Toll-like receptors. Proc. Natl Acad. Sci. USA 102, 9577–9582 (2005).
Lee, B. L. et al. UNC93B1 mediates differential trafficking of endosomal TLRs. eLife 2, e00291 (2013).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
He, X., Jing, Z. & Cheng, G. MicroRNAs: new regulators of Toll-like receptor signaling pathways. Biomed. Res. Int. 2014, 945169 (2014).
Aksoy, E. et al. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat. Immunol. 13, 1045–1054 (2012).
Hornef, M. W., Normark, B. H., Vandewalle, A. & Normark, S. Intracellular recognition of lipopolysaccharide by Toll-like receptor 4 in intestinal epithelial cells. J. Exp. Med. 198, 1225–1235 (2003).
Radstake, T. R. et al. Expression of Toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-γ. Arthritis Rheum. 50, 3856–3865 (2004).
Kondo, T., Kawai, T. & Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 33, 449–458 (2012).
Miggin, S. M. & O'Neill, L. A. New insights into the regulation of TLR signaling. J. Leukoc. Biol. 80, 220–226 (2006).
Horai, R. et al. TNF-α is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J. Clin. Invest. 114, 1603–1611 (2004).
O'Neill, L. A. & Fitzgerald, K. A. & Bowie, A. G. The Toll–IL-1 receptor adaptor family grows to five members. Trends Immunol. 24, 286–290 (2003).
Lin, S. C., Lo, Y. C. & Wu, H. Helical assembly in the MyD88–IRAK4–IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890 (2010).
Drexler, S. K. et al. SIGIRR/TIR-8 is an inhibitor of Toll-like receptor signaling in primary human cells and regulates inflammation in models of rheumatoid arthritis. Arthritis Rheum. 62, 2249–2261 (2010).
O'Neill, L. A. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol. Rev. 226, 10–18 (2008).
Sims, J. E. et al. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science 241, 585–589 (1988).
Gay, N. J. & Keith, F. J. Drosophila Toll and IL-1 receptor. Nature 351, 355–356 (1991).
Heguy, A. et al. Internalization and nuclear localization of interleukin 1 are not sufficient for function. Cell Growth Differ. 2, 311–315 (1991).
Heguy, A., Baldari, C. T., Macchia, G., Telford, J. L. & Melli, M. Amino acids conserved in interleukin-1 receptors (IL-1Rs) and the Drosophila toll protein are essential for IL-1R signal transduction. J. Biol. Chem. 267, 2605–2609 (1992).
Kirschning, C. J., Wesche, H., Merrill Ayres, T. & Rothe, M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188, 2091–2097 (1998).
Yang, R. B. et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395, 284–288 (1998).
Movat, H. Z., Burrowes, C. E., Cybulsky, M. I. & Dinarello, C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am. J. Pathol. 129, 463–476 (1987).
Conti, P. et al. Recombinant interleukin 1 and tumor necrosis factor acting in synergy to release thromboxane, 6-KETO-PGF1 alpha and PGE2 by human neutrophils. Scand. J. Rheumatol. Suppl. 75, 318–324 (1988).
Dinarello, C. A. The biology of interleukin 1 and comparison to tumor necrosis factor. Immunol. Lett. 16, 227–231 (1987).
Okusawa, S., Gelfand, J. A., Ikejima, T., Connolly, R. J. & Dinarello, C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J. Clin. Invest. 81, 1162–1172 (1988).
Mandrup-Poulsen, T., Bendtzen, K., Dinarello, C. A. & Nerup, J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J. Immunol. 139, 4077–4082 (1987).
Joosten, L. A., Helsen, M. M., van de Loo, F. A. & van den Berg, W. B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: a comparativestudy using anti-TNFα, anti-IL-1α/β, and IL-1Ra. Arthritis Rheum. 39, 797–809 (1996).
Zwerina, J. et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl Acad. Sci. USA 104, 11742–11747 (2007).
Chen, C. J. et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13, 851–856 (2007).
So, A. et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 62, 3064–3076 (2010).
Joosten, L. A. et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 62, 3237–3248 (2010).
Liu-Bryan, R., Scott, P., Sydlaske, A., Rose, D. M. & Terkeltaub, R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 52, 2936–2946 (2005).
Jin, M. S. et al. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 (2007).
Garlanda, C., Anders, H. J. & Mantovani, A. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 30, 439–446 (2009).
Gu, Y. F. et al. Discovery of the DIGIRR gene from teleost fish: a novel Toll-IL-1 receptor family member serving as a negative regulator of IL-1 signaling. J. Immunol. 187, 2514–2530 (2011).
Smith, D. E. et al. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity 30, 817–831 (2009).
Termeer, C. et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 195, 99–111 (2002).
Hiratsuka, S. et al. The S100A8–serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2007).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Brentano, F., Schorr, O., Gay, R. E., Gay, S. & Kyburz, D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 52, 2656–2665 (2005).
Yu, M. et al. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock 26, 174–179 (2006).
Foell, D., Wittkowski, H. & Roth, J. Mechanisms of disease: a 'DAMP' view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 3, 382–390 (2007).
Sokolove, J., Zhao, X., Chandra, P. E. & Robinson, W. H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 63, 53–62 (2011).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Roelofs, M. F. et al. The expression of Toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of Toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 52, 2313–2322 (2005).
Ospelt, C. et al. Overexpression of Toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 58, 3684–3692 (2008).
Iwahashi, M. et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 50, 1457–1467 (2004).
Huang, Q., Ma, Y., Adebayo, A. & Pope, R. M. Increased macrophage activation mediated through Toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 56, 2192–2201 (2007).
Kowalski, M. L. et al. Increased responsiveness to Toll-like receptor 4 stimulation in peripheral blood mononuclear cells from patients with recent onset rheumatoid arthritis. Mediators Inflamm. 2008, 132732 (2008).
Davis, M. L. et al. Associations of Toll-like receptor (TLR)-4 single nucleotide polymorphisms and rheumatoid arthritis disease progression: an observational cohort study. Int. Immunopharmacol. 24, 346–352 (2015).
Scher, J. U. & Abramson, S. B. The microbiome and rheumatoid arthritis. Nat. Rev. Rheumatol. 7, 569–578 (2011).
Rogier, R., Koenders, M. I. & Abdollahi-Roodsaz, S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J. Immunol. Res. 2015, 527696 (2015).
Saal, J. G. et al. Synovial Epstein–Barr virus infection increases the risk of rheumatoid arthritis in individuals with the shared HLA-DR4 epitope. Arthritis Rheum. 42, 1485–1496 (1999).
Saal, J. G. et al. Persistence of B19 parvovirus in synovial membranes of patients with rheumatoid arthritis. Rheumatol. Int. 12, 147–151 (1992).
Schrijver, I. A., Melief, M. J., Tak, P. P., Hazenberg, M. P. & Laman, J. D. Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 43, 2160–2168 (2000).
van der Heijden, I. M. et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 43, 593–598 (2000).
Joosten, L. A. et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J. Immunol. 171, 6145–6153 (2003).
Abdollahi-Roodsaz, S. et al. Shift from Toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 58, 3753–3764 (2008).
Joosten, L. A. et al. T cell dependence of chronic destructive murine arthritis induced by repeated local activation of Toll-like receptor-driven pathways: crucial role of both interleukin-1β and interleukin-17. Arthritis Rheum. 58, 98–108 (2008).
Abdollahi-Roodsaz, S. et al. Destructive role of myeloid differentiation factor 88 and protective role of TRIF in interleukin-17-dependent arthritis in mice. Arthritis Rheum. 64, 1838–1847 (2012).
Deng, G. M., Nilsson, I. M., Verdrengh, M., Collins, L. V. & Tarkowski, A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 5, 702–705 (1999).
Zare, F. et al. Arthritogenic properties of double-stranded (viral) RNA. J. Immunol. 172, 5656–5663 (2004).
Choe, J. Y., Crain, B., Wu, S. R. & Corr, M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by Toll-like receptor 4 signaling. J. Exp. Med. 197, 537–542 (2003).
Hayashi, T. et al. Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc. Natl Acad. Sci. USA 106, 2764–2769 (2009).
Alzabin, S. et al. Investigation of the role of endosomal Toll-like receptors in murine collagen-induced arthritis reveals a potential role for TLR7 in disease maintenance. Arthritis Res. Ther. 14, R142 (2012).
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Wu, H. J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Yoshitomi, H. et al. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 (2005).
Stone, M., Fortin, P. R., Pacheco-Tena, C. & Inman, R. D. Should tetracycline treatment be used more extensively for rheumatoid arthritis? Metaanalysis demonstrates clinical benefit with reduction in disease activity. J. Rheumatol. 30, 2112–2122 (2003).
Vaahtovuo, J., Munukka, E., Korkeamaki, M., Luukkainen, R. & Toivanen, P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 35, 1500–1505 (2008).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal TH17 cell differentiation. Immunity 40, 594–607 (2014).
Yang, Y. et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510, 152–156 (2014).
de Pablo, P., Chapple, I. L., Buckley, C. D. & Dietrich, T. Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 5, 218–224 (2009).
Mercado, F. B., Marshall, R. I., Klestov, A. C. & Bartold, P. M. Relationship between rheumatoid arthritis and periodontitis. J. Periodontol. 72, 779–787 (2001).
Mikuls, T. R. et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 66, 1090–1100 (2014).
de Aquino, S. G. et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven TH17 response. J. Immunol. 192, 4103–4111 (2014).
Wegner, N. et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62, 2662–2672 (2010).
Quirke, A. M. et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 73, 263–269 (2014).
Mikuls, T. R. et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int. Immunopharmacol. 9, 38–42 (2009).
Lu, M. C. et al. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor α production. Arthritis Rheum. 62, 1213–1223 (2010).
Sacre, S. M. et al. The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am. J. Pathol. 170, 518–525 (2007).
Sacre, S. M. et al. Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J. Immunol. 181, 8002–8009 (2008).
Ultaigh, S. N. et al. Blockade of Toll-like receptor 2 prevents spontaneous cytokine release from rheumatoid arthritis ex vivo synovial explant cultures. Arthritis Res. Ther. 13, R33 (2011).
Abdollahi-Roodsaz, S. et al. Local interleukin-1-driven joint pathology is dependent on Toll-like receptor 4 activation. Am. J. Pathol. 175, 2004–2013 (2009).
Youssef, P. et al. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J. Rheumatol. 26, 2523–2528 (1999).
Shiozawa, K., Hino, K. & Shiozawa, S. Alternatively spliced EDA-containing fibronectin in synovial fluid as a predictor of rheumatoid joint destruction. Rheumatology (Oxford) 40, 739–742 (2001).
Huang, Q. Q. et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J. Immunol. 182, 4965–4973 (2009).
Huang, Q. Q. et al. Glycoprotein 96 perpetuates the persistent inflammation of rheumatoid arthritis. Arthritis Rheum. 64, 3638–3648 (2012).
Chamberlain, N. D. et al. Ligation of TLR7 by rheumatoid arthritis synovial fluid single strand RNA induces transcription of TNFα in monocytes. Ann. Rheum. Dis. 72, 418–426 (2013).
Abdollahi-Roodsaz, S. et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–2967 (2007).
Kim, S. J. et al. Ligation of TLR5 promotes myeloid cell infiltration and differentiation into mature osteoclasts in rheumatoid arthritis and experimental arthritis. J. Immunol. 193, 3902–3913 (2014).
Kokkola, R. et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 48, 2052–2058 (2003).
van Lent, P. L. et al. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann. Rheum. Dis. 67, 1750–1758 (2008).
Zandman-Goddard, G. & Shoenfeld, Y. Infections and SLE. Autoimmunity 38, 473–485 (2005).
Doria, A. et al. Infections as triggers and complications of systemic lupus erythematosus. Autoimmun. Rev. 8, 24–28 (2008).
Rigante, D., Mazzoni, M. B. & Esposito, S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun Rev. 13, 96–102 (2014).
Absher, D. M. et al. Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS Genet. 9, e1003678 (2013).
Marshak-Rothstein, A. & Rifkin, I. R. Immunologically active autoantigens: the role of Toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 25, 419–441 (2007).
Celhar, T. & Fairhurst, A. M. Toll-like receptors in systemic lupus erythematosus: potential for personalized treatment. Front. Pharmacol. 5, 265–272 (2014).
Koh, Y. T. et al. Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells. J. Immunol. 190, 4982–4990 (2013).
Giltiay, N. V. et al. Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells. J. Exp. Med. 210, 2773–2789 (2013).
Sisirak, V. et al. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 211, 1969–1976 (2014).
Reed, J. H., Sim, S., Wolin, S. L., Clancy, R. M. & Buyon, J. P. Ro60 requires Y3 RNA for cell surface exposure and inflammation associated with cardiac manifestations of neonatal lupus. J. Immunol. 191, 110–116 (2013).
Hwang, S. H. et al. B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. J. Immunol. 189, 5786–5796 (2012).
Avalos, A. M., Busconi, L. & Marshak-Rothstein, A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity 43, 76–83 (2010).
Means, T. K. et al. Human lupus autoantibody–DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115, 407–417 (2005).
Christensen, S. R. et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 202, 321–331 (2005).
Lartigue, A. et al. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J. Immunol. 177, 1349–1354 (2006).
Nickerson, K. M. et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 184, 1840–1848 (2010).
Leadbetter, E. A. et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Nündel, K. et al. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J. Immunol. 194, 2504–2512 (2015).
Desnues, B. et al. TLR8 on dendritic cells and TLR9 on B cells restrain TLR7-mediated spontaneous autoimmunity in C57BL/6 mice. Proc. Natl Acad. Sci. USA 111, 1497–1502 (2014).
Terkeltaub, R. Update on gout: new therapeutic strategies and options. Nat. Rev. Rheumatol. 6, 30–38 (2010).
Rock, K. L., Kataoka, H. & Lai, J. J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 9, 13–23 (2013).
Mylona, E. E. et al. Enhanced interleukin-1β production of PBMCs from patients with gout after stimulation with Toll-like receptor-2 ligands and urate crystals. Arthritis Res. Ther. 14, R158 (2012).
Chen, C. J. et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J. Clin. Invest. 116, 2262–2271 (2006).
Qing, Y. F. et al. Association of TLR4 gene rs2149356 polymorphism with primary gouty arthritis in a case–control study. PLoS ONE 8, e64845 (2013).
Di Giovine, F. S., Malawista, S. E., Nuki, G. & Duff, G. W. Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal-induced IL 1. J. Immunol. 138, 3213–3218 (1987).
Guerne, P. A., Terkeltaub, R., Zuraw, B. & Lotz, M. Inflammatory microcrystals stimulate interleukin-6 production and secretion by human monocytes and synoviocytes. Arthritis Rheum. 32, 1443–1452 (1989).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Holzinger, D. et al. Myeloid-related proteins 8 and 14 contribute to monosodium urate monohydrate crystal-induced inflammation in gout. Arthritis Rheumatol. 66, 1327–1339 (2014).
Crisan, T. O. et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 75, 755–762 (2016).
Alexopoulou, L. et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8, 878–884 (2002).
Oosting, M. et al. TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes. PLoS ONE 6, e25998 (2011).
Schroder, N. W. et al. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J. Immunol. 175, 2534–2540 (2005).
Cervantes, J. L. et al. Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-β. Proc. Natl Acad. Sci. USA 108, 3683–3688 (2011).
Wooten, R. M. et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168, 348–355 (2002).
Bolz, D. D. et al. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173, 2003–2010 (2004).
Oosting, M. et al. Recognition of Borrelia burgdorferi by NOD2 is central for the induction of an inflammatory reaction. J. Infect. Dis. 201, 1849–1858 (2010).
Kuznik, A. et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 186, 4794–4804 (2011).
Jessop, J. D. et al. A long-term five-year randomized controlled trial of hydroxychloroquine, sodium aurothiomalate, auranofin and penicillamine in the treatment of patients with rheumatoid arthritis. Br. J. Rheumatol. 37, 992–1002 (1998).
Park, S. J., Lee, A. N. & Youn, H. S. TBK1-targeted suppression of TRIF-dependent signaling pathway of Toll-like receptor 3 by auranofin. Arch. Pharm. Res. 33, 939–945 (2010).
Costello, D. A., Carney, D. G. & Lynch, M. A. α-TLR2 antibody attenuates the Aβ-mediated inflammatory response in microglia through enhanced expression of SIGIRR. Brain Behav. Immun. 46, 70–79 (2015).
Reilly, M. et al. Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin. Pharmacol. Ther. 94, 593–600 (2013).
van Eden, W. XToll, a recombinant chaperonin 10 as an anti-inflammatory immunomodulator. Curr. Opin. Investig. Drugs 9, 523–533 (2008).
Vanags, D. et al. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet 368, 855–863 (2006).
Novimmune. Novimmune reports successful completion of Phase I trial for NI-0101. [online], http://www.novimmune.com/news/pr140805.html (2014).
Sanchez-Pernaute, O. et al. Citrullination enhances the pro-inflammatory response to fibrin in rheumatoid arthritis synovial fibroblasts. Ann. Rheum. Dis. 72, 1400–1406 (2013).
Divanovic, S. et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat. Immunol. 6, 571–578 (2005).
Thwaites, R., Chamberlain, G. & Sacre, S. Emerging role of endosomal Toll-like receptors in rheumatoid arthritis. Front. Immunol. 5, 1 (2014).
Guiducci, C. et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465, 937–941 (2010).
Ostrach, M. Dynavax regains full rights to investigational TLR 7/9 inhibitor DV1179 following expiration of collaboration with GSK. Dynavax [online], http://investors.dynavax.com/releasedetail.cfm?releaseid=885172 (2014).
Zhu, F. G. et al. A novel antagonist of Toll-like receptors 7, 8 and 9 suppresses lupus disease-associated parameters in NZBW/F1 mice. Autoimmunity. 46, 419–428 (2013).
Arcudi, L. Idera pharmaceuticals announces results of Phase 1 clinical trial of IMO-8400, Toll-like receptor antagonist drug candidate for autoimmune and inflammatory diseases. Idera [online], http://ir.iderapharma.com/phoenix.zhtml?c=208904&p=irol-newsArticle&ID=1834174 (2013).
Sacre, S., Medghalchi, M., Gregory, B., Brennan, F. & Williams, R. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit Toll-like receptors. Arthritis Rheum. 62, 683–693 (2010).
Kenny, E. F. et al. Bruton's tyrosine kinase mediates the synergistic signalling between TLR9 and the B cell receptor by regulating calcium and calmodulin. PLoS ONE 8, e74103 (2013).
Mina-Osorio, P. et al. Suppression of glomerulonephritis in lupus-prone NZB × NZW mice by RN486, a selective inhibitor of Bruton's tyrosine kinase. Arthritis Rheum. 65, 2380–2391 (2013).
Ramirez-Ortiz, Z. G. et al. The receptor TREML4 amplifies TLR7-mediated signaling during antiviral responses and autoimmunity. Nat. Immunol. 16, 495–504 (2015).
Rice, T. W. et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 38, 1685–1694 (2010).
Cheng, K., Wang, X. & Yin, H. Small-molecule inhibitors of the TLR3/dsRNA complex. J. Am. Chem. Soc. 133, 3764–3767 (2011).
Mistry, P. et al. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc. Natl Acad. Sci. USA 112, 5455–5460 (2015).
DeVry, C. G. et al. RDP58, a novel immunomodulatory peptide, ameliorates clinical signs of disease in the Lewis rat model of acute experimental autoimmune encephalomyelitis. J. Neuroimmunol. 152, 33–43 (2004).
Iyer, S. et al. Inhibition of tumor necrosis factor mRNA translation by a rationally designed immunomodulatory peptide. J. Biol. Chem. 275, 17051–17057 (2000).
Holtmann, M. M. RDP-58 (SangStat Medical). IDrugs 6, 1188–1194 (2003).
Capolunghi, F. et al. Pharmacological inhibition of TLR9 activation blocks autoantibody production in human B cells from SLE patients. Rheumatology (Oxford) 49, 2281–2289 (2010).
Nimbus Discovery. IRAK4 inhibitors for the treatment of rheumatic diseases. Nimbus Therapeutics [online], http://www.nimbustx.com/news-events/press-releases/irak4-inhibitors-treatment-rheumatic-diseases (2012).
Bahia, M. S., Kaur, M., Silakari, P. & Silakari, O. Interleukin-1 receptor associated kinase inhibitors: potential therapeutic agents for inflammatory- and immune-related disorders. Cell. Signal. 27, 1039–1055 (2015).
Boone, D. L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Ramachandran, I. R. et al. The phosphatase Src homology region 2 domain-containing phosphatase-1 is an intrinsic central regulator of dendritic cell function. J. Immunol. 186, 3934–3945 (2011).
Carty, M. et al. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 7, 1074–1081 (2006).
Dolan, J. et al. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics 8, 320 (2007).
Gardet, A. et al. LRRK2 is involved in the IFN-γ response and host response to pathogens. J. Immunol. 185, 5577–5585 (2010).
Liu, J. et al. Identification and characterization of a unique leucine-rich repeat protein (LRRC33) that inhibits Toll-like receptor-mediated NF-κB activation. Biochem. Biophys. Res. Commun. 434, 28–34 (2013).
O'Neill, L. A., Golenbock, D. & Bowie, A. G. The history of Toll-like receptors — redefining innate immunity. Nat. Rev. Immunol. 13, 453–460 (2013).
Opal, S. M. et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309, 1154–1162 (2013).
Liu, W., Deyoung, B. R., Chen, X., Evanoff, D. P. & Luo, Y. RDP58 inhibits T cell-mediated bladder inflammation in an autoimmune cystitis model. J. Autoimmun. 30, 257–265 (2008).
Acknowledgements
S.A.-R. is supported by grants 15-A0-00-004310-01 from the Arthritis National Research Foundation (ANRF), VENI grant 916.12.039 from the Netherlands Organization for Scientific Research and AFS 14-1-291 grant from the Dutch Arthritis Foundation.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and made substantial contributions to discussion of the content, writing and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Joosten, L., Abdollahi-Roodsaz, S., Dinarello, C. et al. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol 12, 344–357 (2016). https://doi.org/10.1038/nrrheum.2016.61
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.61
This article is cited by
-
SIGIRR-caspase-8 signaling mediates endothelial apoptosis in Kawasaki disease
Italian Journal of Pediatrics (2023)
-
MyD88 dimerization inhibitor ST2825 targets the aggressiveness of synovial fibroblasts in rheumatoid arthritis patients
Arthritis Research & Therapy (2023)
-
Toll-like receptor 3 activation promotes joint degeneration in osteoarthritis
Cell Death & Disease (2022)
-
Effects of chitosan nanoparticles loaded with mesenchymal stem cell conditioned media on gene expression in Vibrio cholerae and Caco-2 cells
Scientific Reports (2022)
-
Platelet CXCL4 mediates neutrophil extracellular traps formation in ANCA-associated vasculitis
Scientific Reports (2021)