Abstract
Immunotherapeutic interventions have long been utilized in urologic oncology for the treatment of metastatic renal cell or superficial transitional cell carcinoma. Most recently, the first active specific immunotherapeutic approach, a cancer vaccine, has passed the final phase of human testing and its approval by the FDA is pending. However, evidence suggests that the full protective and therapeutic potential of cancer vaccines has not yet been achieved. Through multiple mechanisms, tumors promote conditions in the tumor-bearing host that mitigate or even eliminate the vaccine-induced antitumor response. Restoration of the impaired immune function is, therefore, imperative for achieving optimum vaccine efficacy. Targeted pharmacological interventions are capable of overcoming tumor-mediated immunosuppression, and thereby enable cancer vaccination to reach its full therapeutic potential.
Key Points
-
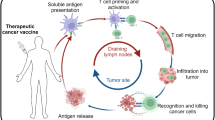
Cancer vaccines are designed to stimulate an antitumor immune response via expansion of the cellular arm of the immune system, especially T cells or natural killer cells
-
At present, the therapeutic effect of cancer vaccines in patients with advanced genitourinary cancers is limited
-
In patients with cancer, tumors promote immunosuppression via multiple mechanisms including secretion of tumor-derived factors, mobilization of bone-marrow-derived suppressor myeloid cells and induction of T-regulatory cells
-
An immunosuppressive microenvironment helps cancer cells to evade immune recognition and immune-mediated destruction
-
Simultaneous targeting of tumor-induced immune suppression and administration of a cancer vaccine has great potential to boost the antitumor immune response and produce a more-powerful therapeutic effect than vaccination alone
-
Clinical trials that are investigating combinatorial approaches that entail abrogation of tumor-induced immunosuppression followed by active immunotherapy are ongoing in many academic and industry programs
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vieweg, J. Immunotherapy for advanced prostate cancer. Rev. Urol. 9 (Suppl. 1), S29–S38 (2007).
Schellhammer, P. F. et al. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC) [abstract LBA9]. Presented at the American Urological Association Annual Meeting [online], (2009).
Vieweg, J. Future directions for vaccine-based therapies. Urol. Oncol. 24, 448–455 (2006).
Vieweg, J. & Dannull, J. Technology Insight: vaccine therapy for prostate cancer. Nat. Clin. Pract. Urol. 2, 44–51 (2005).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2005).
Pardoll, D. & Allison, J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat. Med. 10, 887–892 (2004).
Gilboa, E. The promise of cancer vaccines. Nat. Rev. Cancer 4, 401–411 (2004).
Steinman, R. M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5, 253–274 (2005).
Vieweg, J., Su, Z., Dahm, P. & Kusmartsev, S. Reversal of tumor-induced immune suppression. Clin. Cancer Res. 13, 727–732 (2007).
Rabinovich, G. A., Sotomayor, E. M. & Gabrilovich, D. I. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296 (2007).
Dunn, G., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Willimsky, G. & Blankenstein, T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 437, 141–146 (2005).
Small, E. et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) [abstract 7]. Presented at the Genitourinary Cancers Symposium, American Society of Clinical Oncology (2009).
Brandau, S. Local and systemic immune suppression in bladder cancer. J. Urol. 177, 12–13 (2007).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Murdoch, C., Muthana, M., Coffelt, S. B. & Lewis, C. E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 8, 618–631 (2008).
Almand, B. et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689 (2001).
Kusmartsev, S. et al. Reversal of myeloid cell-mediated immunosuppression on patients with metastatic renal cell carcinoma. Clin. Cancer Res. 14, 8270–8278 (2008).
Ostrand-Rosenberg, S. & Sinha, P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182, 4499–4506 (2009).
Schmielau, J. & Finn, O. J. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 61, 4756–4760 (2001).
Rodriguez, P. C. et al. Arginase I–producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69, 1553–1560 (2009).
Rodriguez, P. C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Kusmartsev, S. & Gabrilovich, D. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 174, 4880–4891 (2005).
Bronte, V. et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201, 1257–1268 (2005).
Kusmartsev, S. et al. Oxidative stress up-regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 181, 346–353 (2008).
Deerwish, I. H., Tannebaum, C. S., Rayman, P. A. & Finke, J. H. Mechanisms of immune dysfunction in renal cell carcinoma. Cancer Treat. Res. 116, 29–51 (2003).
Nagaraj, S. et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835 (2007).
Zea, A. et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65, 3044–3048 (2005).
Serafini, P., Mgebroff, S., Noonan, K. & Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 68, 5439–5449 (2008).
Shevach, E. M., McHugh, R. S., Piccirillo, C. A. & Thornton, A. M. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 182, 58–67 (2001).
Dannull, J. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115, 3623–3633 (2005).
Curiel, T. J. Regulatory T cells and treatment of cancer. Curr. Opin. Immunol. 20, 241–246 (2008).
Christensen, J. G. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann. Oncol. 18 (Suppl. 10), x3–x10 (2007).
Ko, J. S. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 (2009).
Finke, J. H. et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin. Cancer Res. 14, 6674–6682 (2008).
Ozao-Choy, J. et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 69, 2514–2522 (2009).
Xin, H. et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 69, 2506–2513 (2009).
Nefedova, Y. et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 172, 464–474 (2004).
Wang, D. & Dubois, R. N. Prostaglandins and cancer. Gut 55, 115–122 (2006).
Dannenberg, A. & Subbaramaiah, K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 4, 431–436 (2003).
Tai, H. H., Cho, H., Tong, M. & Ding, Y. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological functions. Curr. Pharm. Des. 12, 955–962 (2006).
Eruslanov, E. et al. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor-infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J. Immunol. 182, 7548–7557 (2009).
Fulton, A. M., Ma, X. & Kundu, N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res. 66, 9794–9797 (2006).
Harris, R. E., Beebe-Donk, J. & Alshafie, G. A. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer 8, 237 (2008).
Cuzick, J. et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 10, 501–507 (2009).
Sharma, S. et al. Cyclooxygenase 2 inhibition promotes IFN-γ-dependent enhancement of antitumor responses. J. Immunol. 175, 813–819 (2005).
Sombroek, C. C. et al. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J. Immunol. 168, 4333–4343 (2002).
Stolina, M. et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J. Immunol. 164, 361–370 (2000).
Yang, L. et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J. Clin. Invest. 111, 727–735 (2003).
Talmadge, J. et al. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int. Immunopharmacol. 7, 140–151 (2006).
Sinha, P., Clements, V. K., Fulton, A. M. & Ostrand-Rosenberg, S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 67, 4507–4513 (2007).
DeLong, P. et al. Use of cyclooxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res. 63, 7845–7852 (2003).
Haas, A. R. et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin. Cancer Res. 12, 214–222 (2006).
De Santo, C. et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination Proc. Natl Acad. Sci. USA 102, 4185–4190 (2005).
Mirza, N. et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66, 9299–9307 (2006).
Dowd, N. P., Scully, M., Adderley, S. R., Cunningham, A. J. & Fitzgerald, D. J. Inhibition of cyclooxygenase-2 aggravates doxorubicin-mediated cardiac injury in vivo. J. Clin. Invest. 108, 585–590 (2001).
Levesque, L. E., Brophy, J. M. & Zhang, B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults Ann. Intern. Med. 142, 481–489 (2005).
Rodriguez, P. C. & Ochoa, A. C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 222, 180–191 (2008).
Ochoa, A. C., Zea, A. H., Hernandez, C. & Rodriguez, P. C. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 13, 721s–726s (2007).
Rodriguez, P. C. et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma J. Exp. Med. 202, 931–939 (2005).
Munn, D. H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Katz, J. B., Muller, A. & Prendergast, G. C. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 222, 206–221 (2008).
Mellor, A. L. & Munn, D. H. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol. Today 20, 469–473 (1999).
Löb, S., Königsrainer, A., Rammensee, H. G., Opelz, G. & Terness, P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat. Rev. Cancer 9, 445–452 (2009).
Muller, A. J., DuHadaway, J. B., Donover, P. S., Sutanto-Ward, E. & Prendergast, G. C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11, 312–319 (2005).
Löb, S. et al. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111, 2152–2154 (2008).
Yang, L. et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421 (2004).
Lyden, D. et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7, 1194–1201 (2002).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Ahn, G. O. & Brown, J. M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 13, 193–205 (2008).
Grunewald, M. et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124, 175–189 (2006).
Hattori, K. et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nat. Med. 8, 841–849 (2002).
Ferrara, N. & Kerbel, R. S. Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Wong, D. & Korz, W. Translating an antagonist of chemokine receptor CXCR4: from bench to bedside. Clin. Cancer Res. 14, 7975–7980 (2008).
Luttun, A. et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 8, 831–840 (2002).
Gabrilovich, D. et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92, 4150–4166 (1998).
Gabrilovich, D. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996).
Larrivée, B., Pollet, I. & Karsan, A. Activation of vascular endothelial growth factor receptor-2 in bone marrow leads to accumulation of myeloid cells: role of granulocyte–macrophage colony-stimulating factor. J. Immunol. 175, 3015–3024 (2005).
Gerber, H. P., Condorelli, F., Park, J. & Ferrara, N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem. 272, 23659–23667 (1997).
Sosman, J. & Puzanov, I. Combination targeted therapy in advanced renal cell carcinoma. Cancer 115, 2368–2375 (2009).
Yang, J. C. Bevacizumab for patients with metastatic renal cancer: an update. Clin. Cancer Res. 10, 6367S–6370S (2004).
Rini, B. I. et al. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (Provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer 107, 67–74 (2006).
Vianello, F. et al. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J. Immunol. 176, 2902–2914 (2006).
Kusmartsev, S. et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 63, 4441–4449 (2003).
Suzuki, E., Kappor, V., Jassar, A., Kaizer, L. R. & Albelda, S. M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005).
Hatzimouratidis, K. & Hatzichristou, D. G. Looking to the future for erectile dysfunction therapies. Drugs 68, 231–250 (2008).
Serafini, P. et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 203, 2691–2702 (2006).
Shevach, E. M. Certified professionals: CD4+CD25+ suppressor T cells. J. Exp. Med. 193, F41–F46 (2001).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory cells and immune tolerance. Cell 133, 775–787 (2008).
Sutmuller, R. P. et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 194, 823–832 (2001).
Duvic, M. & Talpur, R. Optimizing denileukin diftitox (Ontak) therapy. Future Oncol. 4, 457–469 (2008).
Ghiringhelli, F. et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 34, 336–344 (2004).
Norian, L. A. et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 69, 3086–3094 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J. Vieweg declares that he has acted as a member of the Advisory Council for CureVac. S. Kusmartsev declares no competing interests.
Rights and permissions
About this article
Cite this article
Kusmartsev, S., Vieweg, J. Enhancing the efficacy of cancer vaccines in urologic oncology: new directions. Nat Rev Urol 6, 540–549 (2009). https://doi.org/10.1038/nrurol.2009.177
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2009.177
This article is cited by
-
Prostate cancer, tumor immunity and a renewed sense of optimism in immunotherapy
Cancer Immunology, Immunotherapy (2012)
-
Prostatakarzinom
Der Urologe (2012)
-
Immunologic mechanisms in RCC and allogeneic renal transplant rejection
Nature Reviews Urology (2010)