Abstract
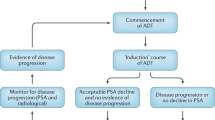
Although androgen deprivation therapy (ADT) has been a cornerstone of the management of prostate cancer for more than 50 years, controversy remains regarding its optimum application. Intermittent androgen suppression (IAS) has been researched since the mid-1980s as a way of reducing the adverse effects and cost of continuous androgen suppression. With preclinical evidence suggesting a potential benefit in terms of time to androgen independence, IAS has been the focus of a number of clinical phase II and III trials. Overall, these trials suggest that IAS is neither inferior nor superior to continuous androgen suppression, with respect to time to castration resistance and cancer-specific survival, but has significant advantages in terms of adverse effects, quality of life and cost. A number of unresolved questions remain, however, including how to select patients for therapy, the optimum duration of therapy, when to restart therapy after the off cycle, and how to define progression to castration-resistant disease. Landmark randomized clinical trials comparing IAS to continuous androgen suppression are in progress and will hopefully answer many of these questions. In future, the use of second-line drugs in the off-treatment phase holds potential for delaying disease progression in men on IAS. At present, men with advanced disease who are deemed candidates for ADT should be informed of IAS as a treatment option, considered experimental from an informed consent point of view, but promising based on current evidence.
Key Points
-
Androgen deprivation therapy is an effective treatment for prostate cancer, but long-term continuous androgen suppression is associated with significant morbidity, high cost, and potentially an increased risk of mortality
-
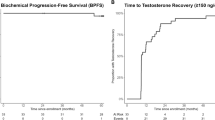
Intermittent androgen suppression (IAS) seems to offer equivalent survival outcomes to continuous suppression, with improved quality of life and reduced cost
-
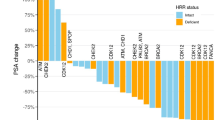
Androgen dependence of prostate cancer can be maintained, although not indefinitely, by cyclic hormone therapy; nadir level of serum PSA is a powerful predictor of time to progression
-
IAS might offer a 'way out' of the immediate versus delayed ADT controversy, by offering the benefits of immediate androgen ablation with reduced treatment-related adverse effects and expense
-
Landmark randomized clinical trials comparing IAS to continuous androgen suppression are in progress and will answer many of the unresolved questions in this field
-
Future improvements to IAS agents and protocols will lengthen the off-treatment time, increase time to castration resistance, and improve prostate-cancer-specific survival
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huggins, C. & Hodges, C. V. Studies on prostatic cancer: effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
Byar, D. P. & Corle, D. K. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group studies. NCI Monogr. 7, 165–170 (1988).
Bruchovsky, N. in Cancer Medicine 3rd edn (eds Holland, J. F., Frei III, E., Bast, R. C., Kufe, D. W., Morton, D. L. & Weichselbaum, R. R.) 884–896 (Lea & Febiger, Philadelphia, 1993).
Braga-Basaria, M. et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J. Clin. Oncol. 24, 3979–3983 (2006).
Mohile, S. G., Mustian, K., Bylow, K., Hall, W. & Dale, W. Management of complications of androgen deprivation therapy in the older man. Crit. Rev. Oncol. Hematol. 70, 235–255 (2009).
Foulds, L. Neoplastic Development, Vol. 1 (Academic Press, New York, 1969).
Noble, R. L. Hormonal control of growth and progression in tumors of Nb rats and a theory of action. Cancer Res. 37, 82–94 (1977).
Bruchovsky, N. et al. Effects of androgen withdrawal on the stem cell composition of Shionogi carcinoma. Cancer Res. 50, 2275–2282 (1990).
Trachtenberg, J. Experimental treatment of prostatic cancer by intermittent hormonal therapy. J. Urol. 137, 785–788 (1987).
Russo, P. et al. Effects of intermittent diethylstilbestrol diphosphate administration on the R3327 rat prostatic carcinoma. Cancer Res. 47, 5967–5970 (1987).
Akakura, K. et al. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer 71, 2782–2790 (1993).
Gleave, M. E., Hsieh, J. T., Wu, H. C., von Eschenbach, A. C. & Chung, L. W. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res. 52, 1598–1605 (1992).
Sato, N. et al. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J. Biol. Chem. 272, 17485–17494 (1997).
Buhler, K. R. et al. Intermittent androgen suppression in the LuCaP 23.12 prostate cancer xenograft model. Prostate 43, 63–70 (2000).
Tsihlias, J. et al. Involvement of p27Kip1 in G1 arrest by high dose 5 alpha-dihydrotestosterone in LNCaP human prostate cancer cells. Oncogene 19, 670–679 (2000).
Vahlensieck, W. & Wegner, G. Continuous versus intermittent oral therapy with estramustine phosphate (Estracyt). Scand. J. Urol. Nephrol. (Suppl.) 55, 147–149 (1980).
Klotz, L. H., Herr, H. W., Morse, M. J. & Whitmore, W. F. Jr. Intermittent endocrine therapy for advanced prostate cancer. Cancer 58, 2546–2550 (1986).
Goldenberg, S. L., Bruchovsky, N., Gleave, M. E., Sullivan, L. D. & Akakura, K. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology 45, 839–844 (1995).
Goldenberg, S. L., Gleave, M. E., Taylor, D. & Bruchovsky, N. Clinical experience with intermittent androgen suppression in prostate cancer: minimum of 3 years' follow-up. Mol. Urol. 3, 287–292 (1999).
Albrecht, W. et al. Intermittent maximal androgen blockade in patients with metastatic prostate cancer: an EORTC feasibility study. Eur. Urol. 44, 505–511 (2003).
Grossfeld, G. D., Small, E. J. & Carroll, P. R. Intermittent androgen deprivation for clinically localized prostate cancer: initial experience. Urology 51, 137–144 (1998).
De La Taille, A. et al. Intermittent androgen suppression in patients with prostate cancer. BJU Int. 91, 18–22 (2003).
Lane, T. M. et al. Long-term outcomes in patients with prostate cancer managed with intermittent androgen suppression. Urol. Int. 73, 117–122 (2004).
Malone, S., Perry, G., Segal, R., Dahrouge, S. & Crook, J. Long-term side-effects of intermittent androgen suppression therapy in prostate cancer: results of a phase II study. BJU Int. 96, 514–520 (2005).
Prapotnich, D. et al. A 10-year clinical experience with intermittent hormonal therapy for prostate cancer. Eur. Urol. 43, 233–239 (2003).
Spry, N. A. et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur. J. Cancer 42, 1083–1092 (2006).
Strum, S. B., Scholz, M. C. & McDermed, J. E. Intermittent androgen deprivation in prostate cancer patients: factors predictive of prolonged time off therapy. Oncologist 5, 45–52 (2000).
Youssef, E., Tekyi-Mensah, S., Hart, K., Bolton, S. & Forman, J. Intermittent androgen deprivation for patients with recurrent/metastatic prostate cancer. Am. J. Clin. Oncol. 26, e119–e123 (2003).
Shaw, G. L. et al. International study into the use of intermittent hormone therapy in the treatment of carcinoma of the prostate: a meta-analysis of 1446 patients. BJU Int. 99, 1056–1065 (2007).
de Leval, J. et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: results of a prospective randomized multicenter trial. Clin. Prostate Cancer 1, 163–171 (2002).
Hussain, M. et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J. Clin. Oncol. 24, 3984–3990 (2006).
Langenhuijsen, J. F. et al. Intermittent androgen suppression in patients with advanced prostate cancer: an update of the TULP survival data [abstract 538]. Eur. Urol. Suppl. 7, 205 (2008).
Miller, K. et al. Randomised prospective study of intermittent versus continuous androgen suppression in advanced prostate cancer [abstract 5015]. J. Clin. Oncol. 25, 2385 (2007).
Schasfoort, E. et al. Intermittent androgen suppression for the treatment of advanced prostate cancer [abstract 1483]. J. Urol. 169 (Suppl.), 397 (2003).
Tunn, U. W. et al. Intermittent is as effective as continuous androgen deprivation in patients with PSA-relapse after radical prostatectomy [abstract 1458]. J. Urol. 171 (Suppl.), 384 (2004).
Verhagen, P. C. M. S. et al. Quality of life effects of intermittent and continuous hormonal therapy by cyproterone acetate (CPA) for metastatic prostate cancer [abstract 541]. Eur. Urol. Suppl. 7, 206 (2008).
Crook, J. et al. A phase III randomized trial comparing intermittent versus continuous androgen suppression for patients with prostate specific antigen progression in the clinical absence of distant metastases following radiotherapy for prostate cancer. http://clinicaltrials.gov/ct2/show/NCT00003653 (2010).
Calais da Silva, F. E. et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur. Urol. 55, 1269–1277 (2009).
Salonen, A. J. et al. Finnish multicenter study comparing intermittent to continuous androgen deprivation for advanced prostate cancer: interim analysis of prognostic markers affecting initial response to androgen deprivation. J. Urol. 180, 915–919 (2008).
Yamanaka, H. et al. Effectiveness of adjuvant intermittent endocrine therapy following neoadjuvant endocrine therapy and external beam radiation therapy in men with locally advanced prostate cancer. Prostate 63, 56–64 (2005).
Hering, F., Rodrigues, P. R. T., Lipay, M. A., Nesrallah, L. & Srougi, M. Metastatic adenocarcinoma of the prostate: comparison between continuous and intermittent hormonal treatment. Int. Braz. J. Urol. 26, 276–282 (2000).
Conti, P. D. et al. Intermittent versus continuous androgen suppression for prostatic cancer. Cochrane Database Syst. Rev. 4, CD005009 (2007).
Abrahamsson, P. A. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur. Urol. 57, 49–59 (2010).
Tangen, C. M. et al. Determinants of prostate specific antigen (PSA) normalization for prostate cancer (PCa) patients (pts) treated with androgen deprivation (AD) on Southwest Oncology Group (SWOG) Study 9346 (INT-0162) [abstract 1591]. Proc. Am. Soc. Clin. Oncol. 22 (2003).
Bruchovsky, N., Klotz, L., Crook, J. & Goldenberg, S. L. Locally advanced prostate cancer—biochemical results from a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen recurrence after radiotherapy. Cancer 109, 858–867 (2007).
Bruchovsky, N. et al. Quality of life, morbidity and mortality results of a prospective phase 2 study of intermittent androgen suppression for men with evidence of prostate-specific antigen relapse after radiation therapy for locally advanced prostate cancer. Clin. Genitourin. Cancer 6, 46–52 (2008).
Kiratli, B. J., Srinivas, S., Perkash, I. & Terris, M. K. Progressive decrease in bone density over 10 years of androgen deprivation therapy in prostate cancer. Urology 57, 127–132 (2000).
Higano, C., Shields, A., Wood, N., Brown, J. & Tangen, C. Bone mineral density in patients with prostate cancer without bone metastases treated with intermittent androgen suppression. Urology 64, 1182–1186 (2004).
Spry, N. A. et al. Long-term effects of intermittent androgen suppression on testosterone recovery and bone mineral density: results of a 33-month observational study. BJU Int. 104, 806–812 (2009).
Hirata, Y., Bruchovsky, N. & Aihara K. Development of a mathematical model that predicts the outcome of hormone therapy for prostate cancer. J. Theor. Biol. 264, 517–527 (2010).
Gleave, M. E, Goldenberg, S. L, Jones, E., Bruchovsky, N. & Sullivan, L. D. Long-term neoadjuvant hormone therapy prior to radical prostatectomy. Analysis of outcome by preoperative risk factors. Mol. Urol. 2, 171–179 (1998).
Miyata, Y., Kanda, S., Sakai, H., Hakariya, T. & Kanetake, H. Relationship between changes in prostate cancer cell proliferation, apoptotic index, and expression of apoptosis-related proteins by neoadjuvant hormonal therapy and duration of such treatment. Urology 65, 1238–1243 (2005).
Laitinen, S., Martikainen, P. M., Tammela, T. L. & Visakorpi, T. Cellular changes in prostate cancer cells induced by intermittent androgen suppression. Eur. Urol. 52, 725–732 (2007).
Scholz, M. et al. Prostate-cancer-ppecific survival and clinical progression-free survival in men with prostate cancer treated intermittently with testosterone-inactivating pharmaceuticals. Urology 70, 506–510 (2007).
Figg, W. D. et al. A double-blind randomized crossover study of oral thalidomide versus placebo for androgen dependent prostate cancer treated with intermittent androgen ablation. J. Urol. 181, 1104–1113 (2009).
Gupta, S. et al. 5a-reductase inhibition during the off cycle of intermittent androgen ablation upregulates tumor suppressive androgen response genes [abstract 539]. J. Urol. 179, 188–189 (2008).
Eggener, S. E. et al. Enhancement of intermittent androgen ablation by “off-cycle” maintenace with finasteride in LNCaP prostate cancer xenograft model. Prostate 66, 495–502 (2006).
Scholz, M. C. et al. Intermittent use of testosterone inactivating pharmaceuticals using finasteride prolongs the time off period. J. Urol. 175, 1673–1678 (2006).
Locke, J. A. & Bruchovsky, N. Prostate cancer: finasteride extends PSA doubling time during intermittent hormone therapy. Can. J. Urol. 17, 5162–5169 (2010).
Klotz, L. & Goldenberg, S. L. Multicenter double-blind study comparing 0.5 mg dutasteride versus placebo daily in men receiving intermittent androgen ablation therapy for prostate cancer (“AVIAS”). http://clinicaltrials.gov/ct2/show/NCT00553878 (2010).
Heidenreich, A. et al. EAU guidelines on prostate cancer. Eur. Urol. 53, 68–80 (2008).
National Institute for Health and Clinical Excellence. Prostate cancer: diagnosis and treatment, [online] (2010).
The National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prostate Cancer-v.3.2010, [online] (2010).
So, A. I., Bowden, M. & Gleave, M. Effect of time of castration and tumour volume on time to androgen-independent recurrence in Shionogi tumours. BJU Int. 93, 845–850 (2004).
Byar, D. P. The Veterans Administration Cooperative Urological Research Group's studies of cancer of the prostate. Cancer 32, 1126–1130 (1973).
Byar, D. P. & Corle, D. K. VACURG randomised trial of radical prostatectomy for stages I and II prostatic cancer. Veterans Administration Cooperative Urology Research Group. Urology 17 (Suppl. 4), 7–11 (1981).
Byar, D. P. & Corle, D. K. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group studies. NCI Monogr. 7, 165–170 (1988).
Medical Research Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the medical research council trial. Br. J. Urol. 79, 235–246 (1997).
Messing, E. M. et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N. Engl. J. Med. 341, 1781–1788 (1999).
McLeod, D. G. et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 97, 247–254 (2006).
Studer, U. E. et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J. Clin. Oncol. 24, 1868–1876 (2006).
Duchesne, G. A collaborative randomised phase III trial: The timing of intervention with androgen deprivation in prostate cancer patients with a rising PSA. http://www.clinicaltrials.gov/ct2/show/NCT00110162 (2010).
Loblaw, A. A randomized comparison of immediate versus deferred androgen deprivation therapy using goserelin for recurrent prostate cancer after radical radiotherapy. http://clinicaltrials.gov/ct2/show/NCT00439751 (2010).
Acknowledgements
Laurie Barclay, Medscape, LLC, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
Both authors were involved in researching data, discussion of content, writing, and review/editing of this article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Buchan, N., Goldenberg, S. Intermittent androgen suppression for prostate cancer. Nat Rev Urol 7, 552–560 (2010). https://doi.org/10.1038/nrurol.2010.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2010.141
This article is cited by
-
Intermittierende Hormontherapie beim androgensensiblen Prostatakarzinom
Der Urologe (2012)
-
Reducing fracture risk in men on androgen deprivation therapy
Nature Reviews Urology (2011)
-
Differing levels of testosterone and the prostate: a physiological interplay
Nature Reviews Urology (2011)
-
An intermittent approach for cancer chemoprevention
Nature Reviews Cancer (2011)