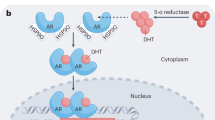
Abstract
It is generally held that the retinoblastoma (RB) tumor suppressor functions in multiple tissues to protect against tumor development. However, preclinical studies and analysis of tumor samples of early disease did not support an important role of RB loss in the origin of prostate cancer. By contrast, recent observations in the clinical setting and subsequent modeling of RB function indicate that the tumor suppressor has specialized roles in controlling androgen receptor expression in prostate cancer, and primarily functions to prevent progression to the castration-resistant stage of disease. Furthermore, preclinical models have now shown that loss of RB expression or functional activity decreases the effectiveness of hormone therapy, yet seems to increase sensitivity to a subset of chemotherapeutic agents. Here, the current state of knowledge regarding the implications of RB loss for prostate cancer progression will be reviewed, and potential opportunities for developing RB as a metric to predict therapeutic response will be considered.
Key Points
-
The retinoblastoma (RB) tumor suppressor is frequently lost or functionally inactivated in castration-resistant prostate cancer
-
RB protects against progression to castration resistance in part through modulation of androgen receptor expression and activity
-
Xenograft models of human prostate cancer suggest that RB downregulation contributes to the emergence of the castration-resistant phenotype
-
Although RB-deficient tumors may respond poorly to hormone therapy, evidence in multiple cancer types suggest that tumors low in RB exhibit a heightened initial response to chemotherapy
-
Tumor cells deficient in RB function show tissue-specific and context-specific loss of DNA damage checkpoints
-
RB status is a candidate for development as a pharmacodynamic marker of transition to castration resistance and as a predictive marker of response to therapy that might guide therapeutic decisions
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal, A., Siegel, R., Xu, J. & Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 (2010).
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Huggins, C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann. Surg. 115, 1192–1200 (1942).
Shen, M. M. & Abate-Shen, C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000 (2010).
Knudsen, K. E. & Penning, T. M. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol. Metab. 21, 315–324 (2010).
Knudsen, B. S. & Vasioukhin, V. Mechanisms of prostate cancer initiation and progression. Adv. Cancer Res. 109, 1–50 (2010).
Gurel, B. et al. Molecular alterations in prostate cancer as diagnostic, prognostic, and therapeutic targets. Adv. Anat. Pathol. 15, 319–331 (2008).
Hansen, M. F. & Cavenee, W. K. Retinoblastoma and the progression of tumor genetics. Trends Genet. 4, 125–128 (1988).
Knudsen, E. S. & Knudsen, K. E. Tailoring to RB: tumour suppressor status and therapeutic response. Nat. Rev. Cancer 8, 714–724 (2008).
Burkhart, D. L. & Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 8, 671–682 (2008).
Bookstein, R. et al. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc. Natl Acad. Sci. USA 87, 7762–7766 (1990).
Kubota, Y. et al. Retinoblastoma gene mutations in primary human prostate cancer. Prostate 27, 314–320 (1995).
Sarkar, F. H. et al. Analysis of retinoblastoma (RB) gene deletion in human prostatic carcinomas. Prostate 21, 145–152 (1992).
Wolff, J. M., Brett, L. T., Lessells, A. M. & Habib, F. K. Analysis of retinoblastoma gene expression in human prostate tissue. Urol. Oncol. 3, 177–182 (1997).
Phillips, S. M. et al. Loss of the retinoblastoma susceptibility gene (RB1) is a frequent and early event in prostatic tumorigenesis. Br. J. Cancer 70, 1252–1257 (1994).
Brooks, J. D., Bova, G. S. & Isaacs, W. B. Allelic loss of the retinoblastoma gene in primary human prostatic adenocarcinomas. Prostate 26, 35–39 (1995).
Ittmann, M. M. & Wieczorek, R. Alterations of the retinoblastoma gene in clinically localized, stage B prostate adenocarcinomas. Hum. Pathol. 27, 28–34 (1996).
Cooney, K. A. et al. Distinct regions of allelic loss on 13q in prostate cancer. Cancer Res. 56, 1142–1145 (1996).
Li, C. et al. Identification of two distinct deleted regions on chromosome 13 in prostate cancer. Oncogene 16, 481–487 (1998).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Sharma, A. et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J. Clin. Invest. 120, 4478–4492 (2010).
Knudsen, E. S. & Wang, J. Y. Targeting the RB-pathway in cancer therapy. Clin. Cancer Res. 16, 1094–1099 (2010).
Bosco, E. E. et al. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J. Clin. Invest. 117, 218–228 (2007).
Markey, M. P. et al. Unbiased analysis of RB-mediated transcriptional repression identifies novel targets and distinctions from E2F action. Cancer Res. 62, 6587–6597 (2002).
Markey, M. P. et al. Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene 26, 6307–6318 (2007).
Herschkowitz, J. I., He, X., Fan, C. & Perou, C. M. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 10, R75 (2008).
Sharma, A. et al. Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells. Cancer Res. 67, 6192–6203 (2007).
Bookstein, R., Shew, J. Y., Chen, P. L., Scully, P. & Lee, W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science 247, 712–715 (1990).
Banerjee, A. et al. Changes in growth and tumorigenicity following reconstitution of retinoblastoma gene function in various human cancer cell types by microcell transfer of chromosome 13. Cancer Res. 52, 6297–6304 (1992).
Maddison, L. A., Sutherland, B. W., Barrios, R. J. & Greenberg, N. M. Conditional deletion of Rb causes early stage prostate cancer. Cancer Res. 64, 6018–6025 (2004).
Wang, Y. et al. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 60, 6008–6017 (2000).
Day, K. C. et al. Rescue of embryonic epithelium reveals that the homozygous deletion of the retinoblastoma gene confers growth factor independence and immortality but does not influence epithelial differentiation or tissue morphogenesis. J. Biol. Chem. 277, 44475–44484 (2002).
Hill, R., Song, Y., Cardiff, R. D. & Van Dyke, T. Heterogeneous tumor evolution initiated by loss of pRb function in a preclinical prostate cancer model. Cancer Res. 65, 10243–10254 (2005).
Zhou, Z. et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 66, 7889–7898 (2006).
Shappell, S. B. et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 64, 2270–2305 (2004).
Knudsen, K. E., Arden, K. C. & Cavenee, W. K. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 273, 20213–20222 (1998).
Fribourg, A. F., Knudsen, K. E., Strobeck, M. W., Lindhorst, C. M. & Knudsen, E. S. Differential requirements for ras and the retinoblastoma tumor suppressor protein in the androgen dependence of prostatic adenocarcinoma cells. Cell Growth Differ. 11, 361–372 (2000).
Libertini, S. J. et al. E2F1 expression in LNCaP prostate cancer cells deregulates androgen dependent growth, suppresses differentiation, and enhances apoptosis. Prostate 66, 70–81 (2006).
Sun, H. et al. E2f binding-deficient Rb1 protein suppresses prostate tumor progression in vivo. Proc. Natl Acad. Sci. USA 108, 704–709 (2011).
Macleod, K. F. The RB tumor suppressor: a gatekeeper to hormone independence in prostate cancer? J. Clin. Invest. 120, 4179–4182 (2010).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Craft, N. et al. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 59, 5030–5036 (1999).
Berges, R. R. et al. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc. Natl Acad. Sci. USA 90, 8910–8914 (1993).
Xu, H. J. et al. Enhanced tumor suppressor gene therapy via replication-deficient adenovirus vectors expressing an N-terminal truncated retinoblastoma protein. Cancer Res. 56, 2245–2249 (1996).
Zhang, X. et al. Adenoviral-mediated retinoblastoma 94 produces rapid telomere erosion, chromosomal crisis, and caspase-dependent apoptosis in bladder cancer and immortalized human urothelial cells but not in normal urothelial cells. Cancer Res. 63, 760–765 (2003).
Pirollo, K. F. et al. Tumor-targeting nanocomplex delivery of novel tumor suppressor RB94 chemosensitizes bladder carcinoma cells in vitro and in vivo. Clin. Cancer Res. 14, 2190–2198 (2008).
Rizzolio, F., Tuccinardi, T., Caligiuri, I., Lucchetti, C. & Giordano, A. CDK inhibitors: from the bench to clinical trials. Curr. Drug Targets 11, 279–290 (2010).
Malumbres, M. & Barbacid, M. Cell cycle kinases in cancer. Curr. Opin. Genet. Dev. 17, 60–65 (2007).
Malumbres, M. Cyclins and related kinases in cancer cells. J. BUON 12 (Suppl. 1), S45–S52 (2007).
Lapenna, S. & Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 8, 547–566 (2009).
O'Dwyer, P. J. et al. A phase I dose escalation trial of a daily oral CDK 4/6 inhibitor PD-0332991. 2007 ASCO Annual Meeting Proceedings. J. Clin. Oncol. 25 (Suppl.), 3550 (2007).
Slamon, D. et al. Phase I study of PD 0332991, cyclin-D kinase (CDK) 4/6 inhibitor in combination with letrozole ofr first-line treatment of patients with ER-positive, HER2-negative breast cancer [abstract 3060]. J. Clin. Oncol. 28 (Suppl.), 15s (2010).
Ertel, A. et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle 9, 4153–4163 (2010).
Konecny, G. E. et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin. Cancer Res. 17, 1591–1602 (2011).
Koh, C. M. et al. Alterations in nucleolar structure and gene expression programs in prostatic neoplasia are driven by the MYC oncogene. Am. J. Pathol. 178, 1824–1834 (2011).
Soucek, L. & Evan, G. I. The ups and downs of Myc biology. Curr. Opin. Genet. Dev. 20, 91–95 (2010).
Hussain, M. et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J. Clin. Oncol. 24, 3984–3990 (2006).
Millikan, R. E. et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J. Clin. Oncol. 26, 5936–5942 (2008).
Rahimi, H. et al. Rb, PTEN and p53 tumor suppressor loss is common in prostatic small cell carcinoma. Presented at the United States & Canadian Academy of Pathology Annual Meeting, San Antonio, TX (2011).
Papandreou, C. N. et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J. Clin. Oncol. 20, 3072–3080 (2002).
Knudsen, K. E. et al. RB-dependent S-phase response to DNA damage. Mol. Cell. Biol. 20, 7751–7763 (2000).
Zagorski, W. A., Knudsen, E. S. & Reed, M. F. Retinoblastoma deficiency increases chemosensitivity in lung cancer. Cancer Res. 67, 8264–8273 (2007).
Trere, D. et al. High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann. Oncol. 20, 1818–1823 (2009).
Pollack, A. et al. Retinoblastoma protein expression and radiation response in muscle-invasive bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 39, 687–695 (1997).
Lassen, P. et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J. Clin. Oncol. 27, 1992–1998 (2009).
Lindel, K., Beer, K. T., Laissue, J., Greiner, R. H. & Aebersold, D. M. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 92, 805–813 (2001).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010).
Chakravarti, A. et al. Prognostic value of p16 in locally advanced prostate cancer: a study based on Radiation Therapy Oncology Group Protocol 9202. J. Clin. Oncol. 25, 3082–3089 (2007).
Chakravarti, A. et al. Loss of p16 expression is of prognostic significance in locally advanced prostate cancer: an analysis from the Radiation Therapy Oncology Group protocol 86–10. J. Clin. Oncol. 21, 3328–3334 (2003).
Den, R. et al. Relationship between the loss of the retinoblastoma tumor suppressor and radiosensitivity [abstract 34]. J. Clin. Oncol. 29 (Suppl. 7), 34 (2011).
Udayakumar, T., Shareef, M. M., Diaz, D. A., Ahmed, M. M. & Pollack, A. The E2F1/Rb and p53/MDM2 pathways in DNA repair and apoptosis: understanding the crosstalk to develop novel strategies for prostate cancer radiotherapy. Semin. Radiat. Oncol. 20, 258–266 (2010).
Acknowledgements
The authors would like to thank Drs Chris Logothetis, Angelo DeMarzo, Adam Dicker, Leonard Gomella, Kevin Kelly, and Erik Knudsen, the Greater Philadelphia Prostate Cancer Working Group, and Matthew Schiewer for critical discussion and comments. We would also like to thank Ms Beth Schade for technical and artwork assistance. We regret omission of additional citations due to space constraints. The authors are supported by grants from the NIH (CA099996 and CA116777 to K. E. Knudsen), US Department of Defense (to R. B. Den), and the Prostate Cancer Foundation (to K. E. Knudsen and R. B. Den).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data, discussing the content and writing the article and performing review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Aparicio, A., Den, R. & Knudsen, K. Time to stratify? The retinoblastoma protein in castrate-resistant prostate cancer. Nat Rev Urol 8, 562–568 (2011). https://doi.org/10.1038/nrurol.2011.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.107
This article is cited by
-
The natural compound atraric acid suppresses androgen-regulated neo-angiogenesis of castration-resistant prostate cancer through angiopoietin 2
Oncogene (2022)
-
Prostate cancer histopathology using label-free multispectral deep-UV microscopy quantifies phenotypes of tumor aggressiveness and enables multiple diagnostic virtual stains
Scientific Reports (2022)
-
UHRF1 overexpression promotes osteosarcoma metastasis through altered exosome production and AMPK/SEMA3E suppression
Oncogenesis (2022)
-
Pharmacologically targetable vulnerability in prostate cancer carrying RB1-SUCLA2 deletion
Oncogene (2020)
-
Flavokawain B targets protein neddylation for enhancing the anti-prostate cancer effect of Bortezomib via Skp2 degradation
Cell Communication and Signaling (2019)