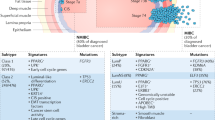
Key Points
-
Mutations in the DNA repair pathway genes ERCC2, FANCC, ATM, and RB1 correlate with complete pathological response to neoadjuvant chemotherapy and should be further evaluated for their utility in clinical decision making
-
Gene mutations, copy number variations, and rearrangements in receptor tyrosine kinase (RTK)– MAPK and PI3K–MTOR pathways are found in ∼70% of bladder tumours; recent studies show that these alterations, in addition to having prognostic significance, can be predictive of response
-
Several ongoing, large pan-cancer trials match patients to a targeted agent (or agents) on the basis of molecular and genetic tumour features, testing for increased efficacy of this personalized medicine approach
-
Immune checkpoint inhibitors that target programmed cell death protein 1 (PD1) and its ligand PDL1 show promise in clinical trials and are approved for bladder cancer treatment; further research is required to personalize the use of these agents
-
Personalized peptide vaccines that are created on the basis of patient leukocyte antigen immune subtypes are currently being tested in clinical trials
Abstract
Effective management of advanced urothelial bladder cancer is challenging. New discoveries that improve our understanding of molecular bladder cancer subtypes have revealed numerous potentially targetable genomic alterations and demonstrated the efficacy of treatments that harness the immune system. These findings have begun to change paradigms of bladder cancer therapy. For example, DNA repair pathway mutations in genes such as ERCC2, FANCC, ATM, RB1, and others can predict responses to neoadjuvant platinum-based chemotherapies and to targeted therapies on the basis of mutation status. Furthermore, an increasing number of pan-cancer clinical trials (commonly referred to as basket or umbrella trials) are enrolling patients on the basis of molecular and genetic predictors of response. These studies promise to provide improved insight into the true utility of personalized medicine in the treatment of bladder cancer and many other cancer types. Finally, therapies that modulate immune responses have shown great benefit in many cancer types. Several immune checkpoint inhibitors that target programmed cell death protein 1 (PD1), its ligand PDL1, and cytotoxic T lymphocyte-associated protein 4 (CTLA4) have already been approved for use in bladder cancer, representing the most important change to the urological oncologist's tool-kit in over a decade. These advances also provide opportunities for personalization of bladder cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Antoni, S. et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur. Urol. 71, 96–108 (2017).
Willis, D. & Kamat, A. M. Nonurothelial bladder cancer and rare variant histologies. Hematol. Oncol. Clin. North Am. 29, 237–252 (2015).
Kamat, A. M. et al. Bladder cancer. Lancet 388, 2796–2810 (2016).
Huncharek, M., Geschwind, J.-F., Witherspoon, B., McGarry, R. & Adcock, D. Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: a meta-analysis of 3703 patients from 11 randomized trials. J. Clin. Epidemiol. 53, 676–680 (2000).
Babjuk, M. et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur. Urol. 71, 447–461 (2017).
Chang, S. S. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 196, 1021–1029 (2016).
Clark, P. E. et al. NCCN guidelines insights: bladder cancer, version 2.2016. J. Natl Compr. Canc. Netw. 14, 1213–1224 (2016).
Svatek, R. S. et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur. Urol. 66, 253–262 (2014).
Milowsky, M. I. et al. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology guideline): American Society of Clinical Oncology Clinical Practice guideline endorsement. J. Clin. Oncol. 34, 1945–1952 (2016).
Witjes, J. A. et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur. Urol. 65, 778–792 (2014).
Jani, A. B., Efstathiou, J. A. & Shipley, W. U. Bladder preservation strategies. Hematol. Oncol. Clin. North Am. 29, 289–300 (2015).
Sternberg, C. N. et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3–pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 16, 76–86 (2015).
Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 361, 1927–1934 (2003).
Winquist, E., Kirchner, T. S., Segal, R., Chin, J. & Lukka, H. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review & meta-analysis. J. Urol. 171, 561–569 (2004).
Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202–205; discussion 205–206 (2005).
David, K. A., Milowsky, M. I., Ritchey, J., Carroll, P. R. & Nanus, D. M. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J. Urol. 178, 451–454 (2007).
Raj, G. V. et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer 117, 276–282 (2011).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003).
International Collaboration of Trialists. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 29, 2171–2177 (2011).
Takata, R. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin. Cancer Res. 11, 2625–2636 (2005).
Jung, Y. & Lippard, S. J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 107, 1387–1407 (2007).
Allen, E. M. V. et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 4, 1140–1153 (2014).
Plimack, E. R. et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur. Urol. 68, 959–967 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01031420 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01611662 (2017).
Liu, D. et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol. 2, 1094–1096 (2016).
Groenendijk, F. H. et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur. Urol. 69, 384–388 (2016).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Damrauer, J. S. et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl Acad. Sci. USA 111, 3110–3115 (2014).
Iyer, G. et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J. Clin. Oncol. 31, 3133–3140 (2013).
Sjödahl, G. et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18, 3377–3386 (2012).
Choi, W. et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Prat, A. et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12, R68 (2010).
Papafotiou, G. et al. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat. Commun. 7, 11914 (2016).
Dadhania, V. et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine 12, 105–117 (2016).
Volkmer, J.-P. et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc. Natl Acad. Sci. USA 109, 2078–2083 (2012).
Warrick, J. I. et al. FOXA1, GATA3 and PPARγ cooperate to drive luminal subtype in bladder cancer: a molecular analysis of established human cell lines. Sci. Rep. 6, 38531 (2016).
McConkey, D. J. et al. Therapeutic opportunities in the intrinsic subtypes of muscle-invasive bladder cancer. Hematol. Oncol. Clin. North Am. 29, 377–394 (2015).
Esserman, L. J. et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res. Treat. 132, 1049–1062 (2012).
Esserman, L. J. et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J. Clin. Oncol. 30, 3242–3249 (2012).
McConkey, D. J., Choi, W. & Dinney, C. P. N. New insights into subtypes of invasive bladder cancer: considerations of the clinician. Eur. Urol. 66, 609–610 (2014).
Kiss, B. et al. Her2 alterations in muscle-invasive bladder cancer: patient selection beyond protein expression for targeted therapy. Sci. Rep. 7, 42713 (2017).
Hoadley, K. A. et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158, 929–944 (2014).
Ikeda, S., Hansel, D. E. & Kurzrock, R. Beyond conventional chemotherapy: emerging molecular targeted and immunotherapy strategies in urothelial carcinoma. Cancer Treat. Rev. 41, 699–706 (2015).
Faltas, B. M. et al. Clonal evolution of chemotherapy-resistant urothelial carcinoma. Nat. Genet. 48, 1490–1499 (2016).
Cha, E. K. et al. Branched evolution and intratumor heterogeneity of urothelial carcinoma of the bladder. J. Clin. Oncol. 32, 293 (2014).
Kim, P. H. et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. Eur. Urol. 67, 198–201 (2015).
Janku, F. et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res. 73, 276–284 (2013).
Rudd, M. L. et al. A unique spectrum of somatic PIK3CA (p110α) mutations within primary endometrial carcinomas. Clin. Cancer Res. 17, 1331–1340 (2011).
Takeuchi, A. et al. p300 mediates cellular resistance to doxorubicin in bladder cancer. Mol. Med. Rep. 5, 173–176 (2012).
Pouessel, D. et al. Tumor heterogeneity of fibroblast growth factor receptor 3 (FGFR3) mutations in invasive bladder cancer: implications for perioperative anti-FGFR3 treatment. Ann. Oncol. 27, 1311–1316 (2016).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Wu, Y.-M. et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 3, 636–647 (2013).
Williams, S. V., Hurst, C. D. & Knowles, M. A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 22, 795–803 (2013).
Sequist, L. V. et al. Phase I study of BGJ398, a selective pan-FGFR inhibitor in genetically preselected advanced solid tumors [abstract]. Cancer Res. 74, CT326 (2014).
Dienstmann, R. et al. First in human study of JNJ-42756493, a potent pan fibroblast growth factor receptor (FGFR) inhibitor in patients with advanced solid tumors. Cancer Res. 74, CT325 (2014).
Lerner, S. P. et al. Summary and recommendations from the National Cancer Institute's clinical trials planning meeting on novel therapeutics for non-muscle invasive bladder cancer. Bladder Cancer 2, 165–202 (2016).
Nogova, L. et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J. Clin. Oncol. 35, 157–165 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01004224 (2017).
Pal, S. K. et al. Efficacy of BGJ398, a fibroblast growth factor receptor (FGFR) 1–3 inhibitor, in patients (pts) with previously treated advanced/metastatic urothelial carcinoma (mUC) with FGFR3 alterations. J. Clin. Oncol. 34, 4517 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01703481 (2017).
Tabernero, J. et al. Phase I dose-escalation study of JNJ-42756493, an oral pan–fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 33, 3401–3408 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02365597 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01732107 (2017).
Hahn, N. M. et al. A phase II trial of dovitinib in Bcg-unresponsive urothelial carcinoma with Fgfr3 mutations or over-expression: Hoosier Cancer Research network trial Hcrn 12–157. Clin. Cancer Res. 23, 3003–3011 (2017).
Chae, Y. K. et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget 8, 16052–16074 (2016).
Herrera-Abreu, M. T. et al. Parallel RNA interference screens identify EGFR activation as an escape mechanism in FGFR3-mutant cancer. Cancer Discov. 3, 1058–1071 (2013).
Wang, L. et al. A functional genetic screen identifies the phosphoinositide 3-kinase pathway as a determinant of resistance to fibroblast growth factor receptor inhibitors in FGFR mutant urothelial cell carcinoma. Eur. Urol. 71, 858–862 (2017).
Datta, J. et al. Akt activation mediates acquired resistance to fibroblast growth factor receptor inhibitor BGJ398. Mol. Cancer Ther. 16, 614–624 (2017).
Baldia, P. H. et al. Fibroblast growth factor receptor (FGFR) alterations in squamous differentiated bladder cancer — a putative therapeutic target for a small subgroup. Oncotarget 7, 71429–71439 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01828736 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00949455 (2015).
Oudard, S. et al. Multicentre randomised phase II trial of gemcitabine + platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur. J. Cancer 51, 45–54 (2015).
Bellmunt, J. et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med. 4, 844–852 (2015).
Powles, T. et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2–positive metastatic bladder cancer. J. Clin. Oncol. 35, 48–55 (2017).
Kim, J. et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Invest. 124, 5145–5158 (2014).
Jordan, E. J. & Iyer, G. Targeted therapy in advanced bladder cancer. Urol. Clin. North Am. 42, 253–262 (2015).
Kurtoglu, M. et al. Elevating the horizon: emerging molecular and genomic targets in the treatment of advanced urothelial carcinoma. Clin. Genitourin. Cancer 13, 410–420 (2015).
Plimack, E. R. & Geynisman, D. M. Targeted therapy for metastatic urothelial cancer: a work in progress. J. Clin. Oncol. 34, 2088–2092 (2016).
Choudhury, N. J. et al. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J. Clin. Oncol. 34, 2165–2171 (2016).
Iyer, G. et al. Genome sequencing identifies a basis for everolimus sensitivity. Science 338, 221 (2012).
Milowsky, M. I. et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 112, 462–470 (2013).
Wagle, N. et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 4, 546–553 (2014).
Prasad, V. & Vandross, A. Characteristics of exceptional or super responders to cancer drugs. Mayo Clin. Proc. 90, 1639–1649 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02243592 (2017).
Poh, A. In search of exceptional responders. Cancer Discov. 5, 8 (2015).
Gui, Y. et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat. Genet. 43, 875–878 (2011).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00421889 (2015).
Faleiro, I. et al. Epigenetic therapy in urologic cancers: an update on clinical trials. Oncotarget 8, 12484–12500 (2016).
Gupta, S. et al. Clinical and translational investigation of pan-HDAC inhibition in advanced urothelial carcinoma (UC). J. Clin. Oncol. 35, 379 (2017).
Helming, K. C., Wang, X. & Roberts, C. W. M. Vulnerabilities of mutant SWI/SNF complexes in cancer. Cancer Cell 26, 309–317 (2014).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00363883 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01780545 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00978250 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02236195 (2017).
Ler, L. D. et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci. Transl. Med. 9, eaai8312 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01042379 (2016).
Park, J. W. et al. Adaptive randomization of neratinib in early breast cancer. N. Engl. J. Med. 375, 11–22 (2016).
Rugo, H. S. et al. Adaptive randomization of veliparib–carboplatin treatment in breast cancer. N. Engl. J. Med. 375, 23–34 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02177695 (2017).
Lee, J. K. et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc. Natl Acad. Sci. USA 104, 13086–13091 (2007).
Smith, S. C. et al. Use of yeast chemigenomics and COXEN informatics in preclinical evaluation of anticancer agents. Neoplasia 13, 72–80 (2011).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02643043 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00851032 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02152254 (2017).
Tsimberidou, A.-M. et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation and landmark analyses. Clin. Cancer Res. 20, 4827–4836 (2014).
Haslem, D. S. et al. A retrospective analysis of precision medicine outcomes in patients with advanced cancer reveals improved progression-free survival without increased health care costs. J. Oncol. Pract. 13, e108–e119 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01566019 (2016).
Massard, C. et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 7, 586–595 (2017).
Le Tourneau, C. et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 16, 1324–1334 (2015).
Tsimberidou, A. M. & Kurzrock, R. Precision medicine: lessons learned from the SHIVA trial. Lancet Oncol. 16, e579–e580 (2015).
Le Tourneau, C., Belin, L., Paoletti, X., Bièche, I. & Kamal, M. Precision medicine: lessons learned from the SHIVA trial — authors' reply. Lancet Oncol. 16, e581–e582 (2015).
Le Tourneau, C. & Kurzrock, R. Targeted therapies: what have we learned from SHIVA? Nat. Rev. Clin. Oncol. 13, 719–720 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02465060 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02693535 (2017).
Doroshow, J. H. Update: NCI formulary & NCI-MATCH trial. National Cancer Institute https://deainfo.nci.nih.gov/advisory/bsa/0317/Doroshow.pdf (2017).
Prasad, V. Perspective: the precision-oncology illusion. Nature 537, S63 (2016).
Togneri, F. S. et al. Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA. Eur. J. Hum. Genet. 24, 1167–1174 (2016).
Guey, L. T. et al. Genetic susceptibility to distinct bladder cancer subphenotypes. Eur. Urol. 57, 283–292 (2010).
Massari, F. et al. Emerging concepts on drug resistance in bladder cancer: implications for future strategies. Crit. Rev. Oncol. Hematol. 96, 81–90 (2015).
Barlow, L. J., Seager, C. M., Benson, M. C. & McKiernan, J. M. Novel intravesical therapies for non-muscle-invasive bladder cancer refractory to BCG. Urol. Oncol. 28, 108–111 (2010).
Fakhrejahani, F. et al. Immunotherapies for bladder cancer: a new hope. Curr. Opin. Urol. 25, 586–596 (2015).
Muthigi, A., George, A. K., Brancato, S. J. & Agarwal, P. K. Novel immunotherapeutic approaches to the treatment of urothelial carcinoma. Ther. Adv. Urol. 8, 203–214 (2016).
Kamat, A. M. et al. Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat. Rev. Urol. 12, 225–235 (2015).
Redelman-Sidi, G., Iyer, G., Solit, D. B. & Glickman, M. S. Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res. 73, 1156–1167 (2013).
Redelman-Sidi, G., Glickman, M. S. & Bochner, B. H. The mechanism of action of BCG therapy for bladder cancer — a current perspective. Nat. Rev. Urol. 11, 153–162 (2014).
Sylvester, R. J., van der Meijden, A. P. M., Witjes, J. A. & Kurth, K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J. Urol. 174, 86–91; discussion 91–92 (2005).
Nepple, K. G., Lightfoot, A. J., Rosevear, H. M., O'Donnell, M. A. & Lamm, D. L. Bacillus calmette-guérin with or without interferon α-2b and megadose versus recommended daily allowance vitamins during induction & maintenance intravesical treatment of nonmuscle invasive bladder cancer. J. Urol. 184, 1915–1919 (2010).
Malmström, P.-U. et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin c versus bacillus calmette-guérin for non–muscle-invasive bladder cancer. Eur. Urol. 56, 247–256 (2009).
Herr, H. W., Milan, T. N. & Dalbagni, G. BCG-refractory versus BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol. Oncol. 33, 108.e1–108.e4 (2015).
Correa, A. F. et al. The role of interferon in the management of BCG refractory nonmuscle invasive bladder cancer. Adv. Urol. 2015, 656918 (2015).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Wong, Y. N. S. et al. Evolving adoptive cellular therapies in urological malignancies. Lancet Oncol. 18, e341–e353 (2017).
Katz, H., Wassie, E. & Alsharedi, M. Checkpoint inhibitors: the new treatment paradigm for urothelial bladder cancer. Med. Oncol. 34, 170 (2017).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Goodnow, C. C., Sprent, J., Fazekas de St Groth, B. & Vinuesa, C. G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435, 590–597 (2005).
Drake, C. G., Jaffee, E. & Pardoll, D. M. Mechanisms of immune evasion by tumors. Adv. Immunol. 90, 51–81 (2006).
Loskog, A. et al. Human bladder carcinoma is dominated by T-regulatory cells and Th1 inhibitory cytokines. J. Urol. 177, 353–358 (2007).
Mohme, M., Riethdorf, S. & Pantel, K. Circulating and disseminated tumour cells – mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 14, 155–167 (2017).
Dyrskjøt, L. et al. Expression of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br. J. Cancer 107, 116–122 (2012).
Sharma, P. et al. Cancer-testis antigens: expression and correlation with survival in human urothelial carcinoma. Clin. Cancer Res. 12, 5442–5447 (2006).
Fradet, Y., Picard, V., Bergeron, A. & LaRue, H. Cancer-testis antigen expression in bladder cancer. Prog. Urol. 16, 421–428 (2006).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02869217 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02457650 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02989064 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01626495 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01029366 (2017).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Bonifant, C. L., Jackson, H. J., Brentjens, R. J. & Curran, K. J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 3, 16011 (2016).
Muranski, P. et al. Increased intensity lymphodepletion and adoptive immunotherapy — how far can we go? Nat. Clin. Pract. Oncol. 3, 668–681 (2006).
Wrzesinski, C. et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J. Immunother. 33, 1–7 (2010).
Klebanoff, C., Khong, H., Antony, P., Palmer, D. & Restifo, N. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 26, 111–117 (2005).
Hunder, N. N. et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 358, 2698–2703 (2008).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03132922 (2017).
Masucci, G. V. et al. Validation of biomarkers to predict response to immunotherapy in cancer: volume I — pre-analytical and analytical validation. J. Immunother. Cancer 4, 76 (2016).
Markham, A. Atezolizumab: first global approval. Drugs 76, 1227–1232 (2016).
Ratner, M. Genentech's PD-L1 agent approved for bladder cancer. Nat. Biotechnol. 34, 789–790 (2016).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02108652 (2017).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Sidaway, P. Bladder cancer: atezolizumab effective against advanced-stage disease. Nat. Rev. Urol. 13, 238 (2016).
[No authors listed.] First-line atezolizumab effective in bladder cancer. Cancer Discov. 6, OF7 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02302807 (2017).
Neuman, T. et al. A harmonization study for the use of 22C3 PD-L1 immunohistochemical staining on ventana's platform. J. Thorac. Oncol. 11, 1863–1868 (2016).
Rebelatto, M. C. et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn. Pathol. 11, 95 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02256436 (2017).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02853305 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02632409 (2017).
Sharma, P. et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 17, 1590–1598 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02387996 (2017).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 18, 312–322 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02897765 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02603432 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02516241 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01693562 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02643303 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02527434 (2017).
Carthon, B. C. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16, 2861–2871 (2010).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01524991 (2017).
Galsky, M. et al. Phase II trial of gemcitabine + cisplatin + ipilimumab in patients with metastatic urothelial cancer. J. Clin. Oncol. 34, 357 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02863913 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01234311 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01513733 (2017).
Ryckman, C., Vandal, K., Rouleau, P., Talbot, M. & Tessier, P. A. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 170, 3233–3242 (2003).
Koike, A. et al. Dynamic mobility of immunological cells expressing S100A8 and S100A9 in vivo: a variety of functional roles of the two proteins as regulators in acute inflammatory reaction. Inflammation 35, 409–419 (2012).
Nakhlé, J. et al. Tasquinimod modulates tumor-infiltrating myeloid cells and improves the antitumor immune response to PD-L1 blockade in bladder cancer. Oncoimmunology 5, e1145333 (2016).
Ribas, A. & Tumeh, P. C. The future of cancer therapy: selecting patients likely to respond to PD1/L1 blockade. Clin. Cancer Res. 20, 4982–4984 (2014).
Drake, C. G., Bivalacqua, T. J. & Hahn, N. M. Programmed cell death ligand-1 blockade in urothelial bladder cancer: to select or not to select. J. Clin. Oncol. 34, 3115–3116 (2016).
Sica, G. L. & Ramalingam, S. S. Assays for PD-L1 expression: do all roads lead to rome? JAMA Oncol. 3, 1058–1059 (2017).
Hirsch, F. R. et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J. Thorac. Oncol. 12, 208–222 (2017).
Yu, H. et al. PD-L1 expression by two complementary diagnostic assays and mRNA in situ hybridization in small cell lung cancer. J. Thorac. Oncol. 12, 110–120 (2017).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Sasada, T. et al. Overcoming the hurdles of randomised clinical trials of therapeutic cancer vaccines. Eur. J. Cancer 46, 1514–1519 (2010).
Sakamoto, S., Noguchi, M., Yamada, A., Itoh, K. & Sasada, T. Prospect and progress of personalized peptide vaccinations for advanced cancers. Expert Opin. Biol. Ther. 16, 689–698 (2016).
Chung, D.-S., Kim, C.-H. & Hong, Y.-K. in Glioma (ed. Yamanaka, R.) 143–150 (Springer, 2012).
Hirayama, M. & Nishimura, Y. The present status and future prospects of peptide-based cancer vaccines. Int. Immunol. 28, 319–328 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02015104 (2017).
Ahmad, S., Lam, T. B. & N'Dow, J. Significance of MUC1 in bladder cancer. BJU Int. 115, 161–162 (2015).
Hegele, A. et al. CA19.9 and CEA in transitional cell carcinoma of the bladder: serological and immunohistochemical findings. Anticancer Res. 30, 5195–5200 (2010).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02010203 (2017).
Keehn, A., Gartrell, B. & Schoenberg, M. P. Vesigenurtacel-L (HS-410) in the management of high-grade nonmuscle invasive bladder cancer. Future Oncol. 12, 2673–2682 (2016).
Steinberg, G. D. et al. Immune response results of vesigenurtacel-l (HS-410) in combination with BCG from a randomized phase II trial in patients with non-muscle invasive bladder cancer (NMIBC). J. Clin. Oncol. 35, 319–319 (2017).
Sasada, T., Yamada, A., Noguchi, M. & Itoh, K. Personalized peptide vaccine for treatment of advanced cancer. Curr. Med. Chem. 21, 2332–2345 (2014).
Matsumoto, K. et al. A phase I study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int. 108, 831–838 (2011).
Noguchi, M. et al. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin. Cancer Res. 22, 54–60 (2016).
Hackl, H., Charoentong, P., Finotello, F. & Trajanoski, Z. Computational genomics tools for dissecting tumour-immune cell interactions. Nat. Rev. Genet. 17, 441–458 (2016).
Aragon-Ching, J. B. & Trump, D. L. Targeted therapies in the treatment of urothelial cancers. Urol. Oncol. 35, 465–472 (2017).
Mendoza, M. C., Er, E. E. & Blenis, J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 36, 320–328 (2011).
Vallo, S. et al. Drug-resistant urothelial cancer cell lines display diverse sensitivity profiles to potential second-line therapeutics. Transl Oncol. 8, 210–216 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02381314 (2017).
Acknowledgements
The authors thank R. T. Jones for his significant input and helpful suggestions to this work and all members of the Theodorescu laboratory.
Author information
Authors and Affiliations
Contributions
K.F. researched data for the article. Both authors made substantial contributions to discussion of the manuscript content and wrote the article. D.T. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.T. is co-founder of Aurora Oncology and has stock options in and is on the scientific advisory boards of Machavert, Precision Profile, and Urogen. D.T. also holds intellectual property (through the University of Colorado) that has been licensed to NantHealth. K.M.F. declares no competing interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Felsenstein, K., Theodorescu, D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol 15, 92–111 (2018). https://doi.org/10.1038/nrurol.2017.179
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.179
This article is cited by
-
How Do Molecular Classifications Affect the Neoadjuvant Treatment of Muscle-Invasive Urothelial Carcinoma?
Molecular Diagnosis & Therapy (2024)
-
Polyamine metabolism patterns characterized tumor microenvironment, prognosis, and response to immunotherapy in colorectal cancer
Cancer Cell International (2023)
-
Molecular classification of high-grade, muscle-invasive urothelial carcinomas and the relationship with CTLA-4 and PD-L1 expression
Surgical and Experimental Pathology (2023)
-
Immune inactivation by VISTA predicts clinical outcome and therapeutic benefit in muscle-invasive bladder cancer
BMC Cancer (2023)
-
An m7G-related lncRNA signature predicts prognosis and reveals the immune microenvironment in bladder cancer
Scientific Reports (2023)