Key Points
-
Lack of appropriate models that recapitulate the complexity and heterogeneity of urological tumours precludes most of the preclinical reagents that enter clinical trials from achieving regulatory approval
-
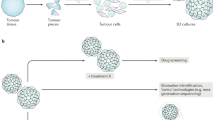
Patient-derived xenograft (PDX) models are characterized by direct engraftment of patient-derived tumour fragments into immunocompromised mice, improving preservation of the identity of the original tumours
-
Use of urological tumour PDX models for analysing tumour biology and, more importantly, in drug development has increased
-
Several agents targeting immune systems have shown promising results in kidney and bladder cancer, but they require PDX models established in mice with an intact immune system to trigger an immune response to the tumour
-
International collaboration for sharing PDX models is a requisite for improving patient outcomes.
Abstract
Lack of appropriate models that recapitulate the complexity and heterogeneity of urological tumours precludes most of the preclinical reagents that target urological tumours from receiving regulatory approval. Patient-derived xenograft (PDX) models are characterized by direct engraftment of patient-derived tumour fragments into immunocompromised mice. PDXs can maintain the original histology, as well as the molecular and genetic characteristics of the source tumour. Thus, PDX models have various advantages over conventional cell-line-derived xenograft (CDX) and other models, which has resulted in an increase in the use of urological tumour PDXs in the analysis of tumour biology and, importantly, for drug development and treatment decisions in personalized medicine. PDX models of urological malignancies have great potential to be used for both basic and clinical research, but limitations exist and need to be overcome. In particular, several agents targeting the immune system have shown promising results in kidney and bladder cancer; however, establishing PDX models in mice with an intact immune system so that an immune response against the tumour is triggered is important to investigate these new therapeutics. Moreover, international collaboration to share PDX models is essential for research concerning fatal urological tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–715 (2004).
Arrowsmith, J. & Miller, P. Trial watch: phase II and phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 12, 569 (2013).
DiMasi, J. A., Reichert, J. M., Feldman, L. & Malins, A. Clinical approval success rates for investigational cancer drugs. Clin. Pharmacol. Ther. 94, 329–335 (2013).
Hidalgo, M. et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 4, 998–1013 (2014).
Tentler, J. J. et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9, 338–350 (2012).
Malaney, P., Nicosia, S. V. & Dave, V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 344, 1–12 (2014).
Morton, C. L. & Houghton, P. J. Establishment of human tumor xenografts in immunodeficient mice. Nat. Protoc. 2, 247–250 (2007).
Michiel Sedelaar, J. P., Dalrymple, S. S. & Isaacs, J. T. Of mice and men — warning: intact versus castrated adult male mice as xenograft hosts are equivalent to hypogonadal versus abiraterone treated aging human males, respectively. Prostate 73, 1316–1325 (2013).
Cho, S. Y. et al. An integrative approach to precision cancer medicine using patient-derived xenografts. Mol. Cells 39, 77–86 (2016).
Feldman, B. J. & Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1, 34–45 (2001).
Kobayashi, T., Inoue, T., Kamba, T. & Ogawa, O. Experimental evidence of persistent androgen-receptor-dependency in castration-resistant prostate cancer. Int. J. Mol. Sci. 14, 15615–15635 (2013).
Shah, R. B. et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 64, 9209–9216 (2004).
Toivanen, R. et al. A preclinical xenograft model identifies castration-tolerant cancer-repopulating cells in localized prostate tumors. Sci. Transl Med. 5, 187ra71 (2013).
Craft, N. et al. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 59, 5030–5036 (1999).
Hoehn, W. et al. Prostatic adenocarcinoma PC EW, a new human tumor line transplantable in nude mice. Prostate 5, 445–452 (1984).
van Weerden, W. M. et al. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am. J. Pathol. 149, 1055–1062 (1996).
van Weerden, W. M. & Romijn, J. C. Use of nude mouse xenograft models in prostate cancer research. Prostate 43, 263–271 (2000).
Pretlow, T. G. et al. Xenografts of primary human prostatic carcinoma. J. Natl Cancer Inst. 85, 394–398 (1993).
Laitinen, S., Karhu, R., Sawyers, C. L., Vessella, R. L. & Visakorpi, T. Chromosomal aberrations in prostate cancer xenografts detected by comparative genomic hybridization. Genes Chromosomes Cancer 35, 66–73 (2002).
True, L. D. et al. A neuroendocrine/small cell prostate carcinoma xenograft-LuCaP 49. Am. J. Pathol. 161, 705–715 (2002).
Klein, K. A. et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat. Med. 3, 402–408 (1997).
Harper, M. E., Goddard, L., Smith, C. & Nicholson, R. I. Characterization of a transplantable hormone-responsive human prostatic cancer xenograft TEN12 and its androgen-resistant sublines. Prostate 58, 13–22 (2004).
McCulloch, D. R., Opeskin, K., Thompson, E. W. & Williams, E. D. BM18: a novel androgen-dependent human prostate cancer xenograft model derived from a bone metastasis. Prostate 65, 35–43 (2005).
Kimura, T. et al. A novel androgen-dependent prostate cancer xenograft model derived from skin metastasis of a Japanese patient. Prostate 69, 1660–1667 (2009).
Lin, D. et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 74, 1272–1283 (2014).
Yoshida, T. et al. Antiandrogen bicalutamide promotes tumor growth in a novel androgen-dependent prostate cancer xenograft model derived from a bicalutamide-treated patient. Cancer Res. 65, 9611–9616 (2005).
Terada, N. et al. Antiandrogen withdrawal syndrome and alternative antiandrogen therapy associated with the W741C mutant androgen receptor in a novel prostate cancer xenograft. Prostate 70, 252–261 (2010).
Hoehn, W., Schroeder, F. H., Reimann, J. F., Joebsis, A. C. & Hermanek, P. Human prostatic adenocarcinoma: some characteristics of a serially transplantable line in nude mice (PC 82). Prostate 1, 95–104 (1980).
Ellis, W. J. et al. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin. Cancer Res. 2, 1039–1048 (1996).
Marques, R. B. et al. The human PC346 xenograft and cell line panel: a model system for prostate cancer progression. Eur. Urol. 49, 245–257 (2006).
Mostaghel, E. A. et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 17, 5913–5925 (2011).
Thadani-Mulero, M. et al. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer Res. 74, 2270–2282 (2014).
Nagabhushan, M. et al. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 56, 3042–3046 (1996).
Nickerson, T. et al. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR). Cancer Res. 61, 6276–6280 (2001).
Seedhouse, S. J. et al. Metastatic phenotype in CWR22 prostate cancer xenograft following castration. Prostate 76, 359–368 (2016).
Corey, E. et al. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate 55, 239–246 (2003).
Chen, C. D. et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 (2004).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Terada, N. et al. Identification of EP4 as a potential target for the treatment of castration-resistant prostate cancer using a novel xenograft model. Cancer Res. 70, 1606–1615 (2010).
Sharma, N. L. et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 23, 35–47 (2013).
Yoshikawa, T. et al. An original patient-derived xenograft of prostate cancer with cyst formation. Prostate 76, 994–1003 (2016).
Li, Z. G. et al. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J. Clin. Invest. 118, 2697–2710 (2008).
Lee, Y. C. et al. Identification of bone-derived factors conferring de novo therapeutic resistance in metastatic prostate cancer. Cancer Res. 75, 4949–4959 (2015).
Small, E. J. et al. Clinical and genomic characterization of metastatic small cell/neuroendocrine prostate cancer (SCNC) and intermediate atypical prostate cancer (IAC): results from the SU2C/PCF/AACRWest Coast Prostate Cancer Dream Team (WCDT) [abstract]. J. Clin. Oncol. 34 (Suppl.), 5019 (2016).
Hirano, D., Okada, Y., Minei, S., Takimoto, Y. & Nemoto, N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur. Urol. 45, 586–592 (2004).
Nadal, R., Schweizer, M., Kryvenko, O. N., Epstein, J. I. & Eisenberger, M. A. Small cell carcinoma of the prostate. Nat. Rev. Urol. 11, 213–219 (2014).
Tzelepi, V. et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin. Cancer Res. 18, 666–677 (2012).
Aparicio, A. et al. Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient's tumor: morphological, immunohistochemical, and gene expression profiles. Prostate 71, 846–856 (2011).
Akamatsu, S. et al. The placental gene PEG10 promotes progression of neuroendocrine prostate cancer. Cell Rep. 12, 922–936 (2015).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Dardenne, E. et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 30, 563–577 (2016).
Lee, J. K. et al. N-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell 29, 536–547 (2016).
Motzer, R. J. et al. Kidney cancer. J. Natl Compr. Canc. Netw. 9, 960–977 (2011).
Sivanand, S. et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci. Transl Med. 4, 137ra75 (2012).
Pavia-Jimenez, A., Tcheuyap, V. T. & Brugarolas, J. Establishing a human renal cell carcinoma tumorgraft platform for preclinical drug testing. Nat. Protoc. 9, 1848–1859 (2014).
Karam, J. A. et al. Development and characterization of clinically relevant tumor models from patients with renal cell carcinoma. Eur. Urol. 59, 619–628 (2011).
Glukhova, L. et al. Overrepresentation of 7q31 and 17q in renal cell carcinomas. Genes Chromosomes Cancer 22, 171–178 (1998).
Schuller, A. G. et al. The MET inhibitor AZD6094 (aavolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient-derived xenograft models. Clin. Cancer Res. 21, 2811–2819 (2015).
Hammers, H. J. et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol. Cancer Ther. 9, 1525–1535 (2010).
Miles, K. M. et al. Dll4 blockade potentiates the anti-tumor effects of VEGF inhibition in renal cell carcinoma patient-derived xenografts. PLoS ONE 9, e112371 (2014).
Adelaiye, R. et al. Sunitinib dose escalation overcomes transient resistance in clear cell renal cell carcinoma and is associated with epigenetic modifications. Mol. Cancer Ther. 14, 513–522 (2015).
Thong, A. E. et al. Tissue slice grafts of human renal cell carcinoma: an authentic preclinical model with high engraftment rate and metastatic potential. Urol. Oncol. 32, 43.e23–43.e30 (2014).
Valta, M. P. et al. Development of a realistic in vivo bone metastasis model of human renal cell carcinoma. Clin. Exp. Metastasis 31, 573–584 (2014).
Yuen, J. S. et al. Inhibition of angiogenic and non-angiogenic targets by sorafenib in renal cell carcinoma (RCC) in a RCC xenograft model. Br. J. Cancer 104, 941–947 (2011).
Grisanzio, C. et al. Orthotopic xenografts of RCC retain histological, immunophenotypic and genetic features of tumours in patients. J. Pathol. 225, 212–221 (2011).
Varna, M. et al. Stability of preclinical models of aggressive renal cell carcinomas. Int. J. Clin. Exp. Pathol. 7, 2950–2962 (2014).
Shibasaki, N. et al. Role of IL13RA2 in sunitinib resistance in clear cell renal cell carcinoma. PLoS ONE 10, e0130980 (2015).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Arakaki, R. et al. CCL2 as a potential therapeutic target for clear cell renal cell carcinoma. Cancer Med. 5, 2920–2933 (2016).
Diaz-Montero, C. M. et al. MEK inhibition abrogates sunitinib resistance in a renal cell carcinoma patient-derived xenograft model. Br. J. Cancer 115, 920–928 (2016).
Lang, H. et al. Establishment of a large panel of patient-derived preclinical models of human renal cell carcinoma. Oncotarget 7, 59336–59359 (2016).
Wood, D. P. in Campbell–Walsh Urology 10th edn 2309–2334 (Saunders, 2012).
Roupret, M. et al. European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur. Urol. 68, 868–879 (2015).
Kamat, A. M. et al. Bladder cancer. Lancet 388, 2796–2810 (2016).
Prasad, S. M., Decastro, G. J. & Steinberg, G. D. Urothelial carcinoma of the bladder: definition, treatment and future efforts. Nat. Rev. Urol. 8, 631–642 (2011).
Sufrin, G., McGarry, M. P., Sandberg, A. A. & Murphy, G. P. Heterotransplantation of human transitional cell carcinoma in athymic mice. J. Urol. 121, 159–161 (1979).
Naito, S. et al. Heterotransplantation of human urinary bladder cancers in nude mice. Invest. Urol. 18, 285–288 (1981).
Matthews, P. N., Grant, A. G. & Hermon-Taylor, J. The growth of human bladder and kidney cancers as xenografts in nude mice and rats. Urol. Res. 10, 293–299 (1982).
Kovnat, A., Armitage, M. & Tannock, I. Xenografts of human bladder cancer in immune-deprived mice. Cancer Res. 42, 3696–3703 (1982).
Bernardo, C., Costa, C., Sousa, N., Amado, F. & Santos, L. Patient-derived bladder cancer xenografts: a systematic review. Transl Res. 166, 324–331 (2015).
Skowron, K. B. et al. Basal tumor cell isolation and patient-derived xenograft engraftment identify high-risk clinical bladder cancers. Sci. Rep. 6, 35854 (2016).
Wei, L. et al. Genomic profiling is predictive of response to cisplatin treatment but not to PI3K inhibition in bladder cancer patient-derived xenografts. Oncotarget 7, 76374–76389 (2016).
Kobayashi, T., Owczarek, T. B., McKiernan, J. M. & Abate-Shen, C. Modelling bladder cancer in mice: opportunities and challenges. Nat. Rev. Cancer 15, 42–54 (2015).
Izquierdo, L., Mengual, L., Gazquez, C., Ingelmo-Torres, M. & Alcaraz, A. Molecular characterization of upper urinary tract tumours. BJU Int. 106, 868–872 (2010).
Sfakianos, J. P. et al. Genomic characterization of upper tract urothelial carcinoma. Eur. Urol. 68, 970–977 (2015).
Russell, P. J. et al. Bladder cancer xenografts: a model of tumor cell heterogeneity. Cancer Res. 46, 2035–2040 (1986).
McCue, P. A. et al. Development of secondary structure, growth characteristics and cytogenetic analysis of human transitional cell carcinoma xenografts in scid/scid mice. J. Urol. 155, 1128–1132 (1996).
Abe, T. et al. Establishment and characterization of human urothelial cancer xenografts in severe combined immunodeficient mice. Int. J. Urol. 13, 47–57 (2006).
Chan, K. S. et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl Acad. Sci. USA 106, 14016–14021 (2009).
Park, B. et al. Development and characterization of a bladder cancer xenograft model using patient-derived tumor tissue. Cancer Sci. 104, 631–638 (2013).
Hofner, T. et al. Development and characteristics of preclinical experimental models for the research of rare neuroendocrine bladder cancer. J. Urol. 190, 2263–2270 (2013).
Ciamporcero, E. et al. YAP activation protects urothelial cell carcinoma from treatment-induced DNA damage. Oncogene 35, 1541–1553 (2015).
Bernardo, C. et al. Patient-derived sialyl-Tn-positive invasive bladder cancer xenografts in nude mice: an exploratory model study. Anticancer Res. 34, 735–744 (2014).
Jager, W. et al. Patient-derived bladder cancer xenografts in the preclinical development of novel targeted therapies. Oncotarget 6, 21522–21532 (2015).
Pan, C. X. et al. Development and characterization of bladder cancer patient-derived xenografts for molecularly guided targeted therapy. PLoS ONE 10, e0134346 (2015).
Kurtova, A. V. et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 517, 209–213 (2015).
Russell, P. J. et al. Features of squamous and adenocarcinoma in the same cell in a xenografted human transitional cell carcinoma: evidence of a common histogenesis? Urol. Res. 16, 79–84 (1988).
Hay, J. H., Busuttil, A., Steel, C. M. & Duncan, W. The growth and histological characteristics of a series of human bladder cancer xenografts. Radiother. Oncol. 7, 331–340 (1986).
Mouse Tumor Biology Database. Patient derived xenograft search form. MTB http://tumor.informatics.jax.org/mtbwi/pdxSearch.do (2016).
Cirone, P., Andresen, C. J., Eswaraka, J. R., Lappin, P. B. & Bagi, C. M. Patient-derived xenografts reveal limits to PI3K/mTOR- and MEK-mediated inhibition of bladder cancer. Cancer Chemother. Pharmacol. 73, 525–538 (2014).
Lawrence, M. G. et al. A preclinical xenograft model of prostate cancer using human tumors. Nat. Protoc. 8, 836–848 (2013).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 7–13 (2016).
van Weerden, W. M., Bangma, C. & de Wit, R. Human xenograft models as useful tools to assess the potential of novel therapeutics in prostate cancer. Br. J. Cancer 100, 13–18 (2009).
DeRose, Y. S. et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 17, 1514–1520 (2011).
Tomayko, M. M. & Reynolds, C. P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 24, 148–154 (1989).
Bosma, M. J. & Carroll, A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu. Rev. Immunol. 9, 323–350 (1991).
Shultz, L. D. et al. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb. Protoc. 2014, 694–708 (2014).
Pearson, T., Greiner, D. L. & Shultz, L. D. Humanized SCID mouse models for biomedical research. Curr. Top. Microbiol. Immunol. 324, 25–51 (2008).
Jackson Laboratory. Patient-derived xenograft (PDX) models. JAX https://www.jax.org/jax-mice-and-services/in-vivo-pharmacology/oncology-services/pdx-tumors (2016).
Shultz, L. D., Brehm, M. A., Garcia-Martinez, J. V. & Greiner, D. L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12, 786–798 (2012).
Cao, X. et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2, 223–238 (1995).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Pearson, T. et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin. Exp. Immunol. 154, 270–284 (2008).
Ito, R., Takahashi, T., Katano, I. & Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 9, 208–214 (2012).
Landis, M. D., Lehmann, B. D., Pietenpol, J. A. & Chang, J. C. Patient-derived breast tumor xenografts facilitating personalized cancer therapy. Breast Cancer Res. 15, 201 (2013).
Shultz, L. D. et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154, 180–191 (1995).
Wang, Y. et al. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 61, 6064–6072 (2001).
Wang, Y. et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate 64, 149–159 (2005).
Saar, M. et al. Orthotopic tumorgrafts in nude mice: a new method to study human prostate cancer. Prostate 75, 1526–1537 (2015).
Jager, W. et al. Ultrasound-guided intramural inoculation of orthotopic bladder cancer xenografts: a novel high-precision approach. PLoS ONE 8, e59536 (2013).
Jager, W. et al. Minimally invasive establishment of murine orthotopic bladder xenografts. J. Vis. Exp. 84, e51123 (2014).
Pearson, A. T. et al. Patient-derived xenograft (PDX) tumors increase growth rate with time. Oncotarget 7, 7993–8005 (2016).
Cooper, C. S. et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat. Genet. 47, 367–372 (2015).
Boutros, P. C. et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet. 47, 736–745 (2015).
Dalgliesh, G. L. et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463, 360–363 (2010).
Morrison, C. D. et al. Whole-genome sequencing identifies genomic heterogeneity at a nucleotide and chromosomal level in bladder cancer. Proc. Natl Acad. Sci. USA 111, E672–E681 (2014).
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21, 1318–1325 (2015).
Bertotti, A. et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 1, 508–523 (2011).
Alkema, N. G. et al. Biobanking of patient and patient-derived xenograft ovarian tumour tissue: efficient preservation with low and high fetal calf serum based methods. Sci. Rep. 5, 14495 (2015).
Cui, J. H., Krueger, U., Henne-Bruns, D., Kremer, B. & Kalthoff, H. Orthotopic transplantation model of human gastrointestinal cancer and detection of micrometastases. World J. Gastroenterol. 7, 381–386 (2001).
Linnebacher, M. et al. Cryopreservation of human colorectal carcinomas prior to xenografting. BMC Cancer 10, 362 (2010).
Sorio, C. et al. Successful xenografting of cryopreserved primary pancreatic cancers. Virchows Arch. 438, 154–158 (2001).
Lin, D. et al. Next generation patient-derived prostate cancer xenograft models. Asian J. Androl. 16, 407–412 (2014).
Goldenberg, D. M. & Pavia, R. A. In vivo horizontal oncogenesis by a human tumor in nude mice. Proc. Natl Acad. Sci. USA 79, 2389–2392 (1982).
Russell, P. J. et al. Tumour-induced host stromal-cell transformation: induction of mouse spindle-cell fibrosarcoma not mediated by gene transfer. Int. J. Cancer 46, 299–309 (1990).
Bondarenko, G. et al. Patient-derived tumor xenografts are susceptible to formation of human lymphocytic tumors. Neoplasia 17, 735–741 (2015).
Wetterauer, C. et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock-out immunocompromised mice. Prostate 75, 585–592 (2015).
Radaelli, E. et al. Spontaneous post-transplant disorders in NOD. Cg- Prkdcscid Il2rgtm1Sug/JicTac (NOG) mice engrafted with patient-derived metastatic melanomas. PLoS ONE 10, e0124974 (2015).
Morote, J. et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J. Urol. 178, 1290–1295 (2007).
Mohr, B. A., Guay, A. T., O'Donnell, A. B. & McKinlay, J. B. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin. Endocrinol. (Oxf.) 62, 64–73 (2005).
van Weerden, W. M., Bierings, H. G., van Steenbrugge, G. J., de Jong, F. H. & Schroder, F. H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 50, 857–861 (1992).
Pandhare-Dash, J., Mantri, C. K., Gong, Y., Chen, Z. & Dash, C. XMRV accelerates cellular proliferation, transformational activity, and invasiveness of prostate cancer cells by downregulating p27(Kip1). Prostate 72, 886–897 (2012).
Hempel, H. A., Burns, K. H., De Marzo, A. M. & Sfanos, K. S. Infection of xenotransplanted human cell lines by murine retroviruses: a lesson brought back to light by XMRV. Front. Oncol. 3, 156 (2013).
Williams, S. A., Anderson, W. C., Santaguida, M. T. & Dylla, S. J. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab. Invest. 93, 970–982 (2013).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Kleb, B. et al. Differentially methylated genes and androgen receptor re-expression in small cell prostate carcinomas. Epigenetics 11, 184–193 (2016).
Lee, Y. P. et al. Use of zoledronate to treat osteoblastic versus osteolytic lesions in a severe-combined-immunodeficient mouse model. Cancer Res. 62, 5564–5570 (2002).
Lee, Y. C. et al. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 71, 5194–5203 (2011).
Lee, Y. C. et al. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol. Cell. Proteomics 14, 471–483 (2015).
Cottu, P. et al. Acquired resistance to endocrine treatments is associated with tumor-specific molecular changes in patient-derived luminal breast cancer xenografts. Clin. Cancer Res. 20, 4314–4325 (2014).
Kreso, A. et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 339, 543–548 (2013).
Bertolini, G. et al. Microenvironment-modulated metastatic CD133+/CXCR4+/EpCAM- lung cancer-initiating cells sustain tumor dissemination and correlate with poor prognosis. Cancer Res. 75, 3636–3649 (2015).
Simoes, B. M. et al. Anti-estrogen resistance in human breast tumors is driven by JAG1-NOTCH4-dependent cancer stem cell activity. Cell Rep. 12, 1968–1977 (2015).
Garralda, E. et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin. Cancer Res. 20, 2476–2484 (2014).
Brugarolas Laboratory. Research program overview. UTSouthwestern http://www3.utsouthwestern.edu/brugarolaslab/research-programs.php (2016).
Wolff, N. C. et al. High-throughput simultaneous screen and counterscreen identifies homoharringtonine as synthetic lethal with von Hippel-Lindau loss in renal cell carcinoma. Oncotarget 6, 16951–16962 (2015).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Azad, A. A. et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin. Cancer Res. 21, 2315–2324 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Rongvaux, A. et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 32, 364–372 (2014).
Yamamoto, K. et al. Stromal remodeling by the BET bromodomain inhibitor JQ1 suppresses the progression of human pancreatic cancer. Oncotarget 7, 61469–61484 (2016).
Calon, A. et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 47, 320–329 (2015).
Isella, C. et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 47, 312–319 (2015).
Fong, E. L. et al. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 77, 164–172 (2016).
Ozkumur, E. et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl Med. 5, 179ra47 (2013).
Yu, M. et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Baccelli, I. et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544 (2013).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Cayrefourcq, L. et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 75, 892–901 (2015).
Williams, E. S. et al. Generation of prostate cancer patient derived xenograft models from circulating tumor cells. J. Vis. Exp. 104, e53182 (2015).
Author information
Authors and Affiliations
Contributions
T.I., N.T., T.K., and O.O. researched the data for the article. All authors substantially contributed to discussion of content, wrote the manuscript, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Supplementary information
Supplementary information S1 (table)
Prostate cancer patient-derived xenograft (PDX) models. (DOC 102 kb)
Supplementary information S2 (table)
Urothelial cancer patient-derived xenograft PDX models. (DOC 148 kb)
Rights and permissions
About this article
Cite this article
Inoue, T., Terada, N., Kobayashi, T. et al. Patient-derived xenografts as in vivo models for research in urological malignancies. Nat Rev Urol 14, 267–283 (2017). https://doi.org/10.1038/nrurol.2017.19
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.19
This article is cited by
-
The future of patient-derived xenografts in prostate cancer research
Nature Reviews Urology (2023)
-
Vector engineering, strategies and targets in cancer gene therapy
Cancer Gene Therapy (2022)
-
The cell-line-derived subcutaneous tumor model in preclinical cancer research
Nature Protocols (2022)
-
Magnesium galvanic cells produce hydrogen and modulate the tumor microenvironment to inhibit cancer growth
Nature Communications (2022)
-
Faithful preclinical mouse models for better translation to bedside in the field of immuno-oncology
International Journal of Clinical Oncology (2020)