Abstract
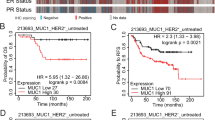
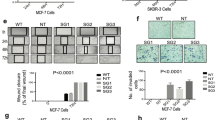
The cancer stem cell hypothesis proposes that cancers arise in stem/progenitor cells through disregulation of self-renewal pathways generating tumors, which are driven by a component of ‘tumor-initiating cells’ retaining stem cell properties. The HER2 gene is amplified in 20–30% of human breast cancers and has been implicated in mammary tumorigenesis as well as in mediating aggressive tumor growth and metastasis. We demonstrate that HER2 overexpression drives mammary carcinogenesis, tumor growth and invasion through its effects on normal and malignant mammary stem cells. HER2 overexpression in normal mammary epithelial cells (NMEC) increases the proportion of stem/progenitor cells as demonstrated by in vitro mammosphere assays and the expression of stem cell marker aldehyde dehydrogenase (ALDH) as well as by generation of hyperplastic lesions in humanized fat pads of NOD (nucleotide-binding oligomerization domain)/SCID (severe combined immunodeficient) mice. Overexpression of HER2 in a series of breast carcinoma cell lines increases the ALDH-expressing ‘cancer stem cell’ population which displays increased expression of stem cell regulatory genes, increased invasion in vitro and increased tumorigenesis in NOD/SCID mice. The effects of HER2 overexpression on breast cancer stem cells are blocked by trastuzumab in sensitive, but not resistant, cell lines, an effect mediated by the PI3-kinase/Akt pathway. These studies provide support for the cancer stem cell hypothesis by suggesting that the effects of HER2 amplification on carcinogenesis, tumorigenesis and invasion may be due to its effects on normal and malignant mammary stem/progenitor cells. Furthermore, the clinical efficacy of trastuzumab may relate to its ability to target the cancer stem cell population in HER2-amplified tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . (2003). Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100: 3983–3988.
Allred DC, O’Connell P, Fuqua SA, Osborne CK . (1994). Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat 32: 13–18.
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K et al. (2007). A functional genetic approach identifies the PI3K pathway as a major determinant of Trastuzumab resistance in breast cancer. Cancer Cell 12: 395–402.
Beuzeboc P, Scholl S, Garau XS, Vincent-Salomon A, Cremoux PD, Couturier J et al. (1999). Herceptin, a monoclonal humanized antibody anti-HER2: a major therapeutic progress in breast cancers overexpressing this oncogene? Bull Cancer 86: 544–549.
Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM et al. (2001). Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 19: 2722–2730.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ et al. (2003). In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17: 1253–1270.
Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS . (2004). Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res 6: R605–R615.
Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N et al. (2007). Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/‘triple-negative’ breast cancer cell lines growing in vitro. Breast Cancer Res Treat 105: 319–326.
Ginestier C, Hur M, Charafe-Jauffret E, Monville F, Dutcher J, Brown M et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and predictor of poor clinical outcome. Cell Stem Cell 1: 555–567.
Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L et al. (2003). HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 22: 3205–3212.
Korkaya H, Wicha MS . (2007). Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs 21: 299–310.
Kucab JE, Lee C, Chen CS, Zhu J, Gilks CB, Cheang M et al. (2005). Celecoxib analogues disrupt Akt signaling, which is commonly activated in primary breast tumours. Breast Cancer Res 7: R796–R807.
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu M-F et al. (2008). Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100: 672–679.
Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW et al. (2006). Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66: 6063–6071.
Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY . (2007). CD133(+) HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 27: 1749–1758.
Miller KD . (2004). The role of ErbB inhibitors in trastuzumab resistance. Oncologist 9 (Suppl 3): 16–19.
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA et al. (2004). PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6: 117–127.
Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C et al. (1990). Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol 8: 103–112.
Park K, Han S, Kim HJ, Kim J, Shin E . (2006). HER2 status in pure ductal carcinoma in situ and in the intraductal and invasive components of invasive ductal carcinoma determined by fluorescence in situ hybridization and immunohistochemistry. Histopathology 48: 702–707.
Phillips TM, McBride WH, Pajonk F . (2006). The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 98: 1777–1785.
Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M et al. (2007). Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA 104: 7564–7569.
Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A et al. (2001). Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 19: 2587–2595.
Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH et al. (2006). CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 8: R59.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL . (1987). Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712.
Stingl J, Eaves CJ, Kuusk U, Emerman JT . (1998). Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63: 201–213.
Tanner M, Kapanen AI, Junttila T, Raheem O, Grenman S, Elo J et al. (2004). Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther 3: 1585–1592.
Tokuzawa Y, Kaiho E, Maruyama M, Takahashi K, Mitsui K, Maeda M et al. (2003). Fbx15 is a novel target of Oct3/4 but is dispensable for embryonic stem cell self-renewal and mouse development. Mol Cell Biol 23: 2699–2708.
Welham MJ, Storm MP, Kingham E, Bone HK . (2007). Phosphoinositide 3-kinases and regulation of embryonic stem cell fate. Biochem Soc Trans 35: 225–228.
Wicha MS, Liu S, Dontu G . (2006). Cancer stem cells: an old idea—a paradigm shift. Cancer Res 66: 1883–1890; discussion 1895–6.
Xu R, Perle MA, Inghirami G, Chan W, Delgado Y, Feiner H . (2002). Amplification of Her-2/neu gene in Her-2/neu-overexpressing and -nonexpressing breast carcinomas and their synchronous benign, premalignant, and metastatic lesions detected by FISH in archival material. Mod Pathol 15: 116–124.
Acknowledgements
We would like to thank Dr Thomas Giordano and the University of Michigan Cancer Center Flow Cytometry core for their assistance, Dr Bruce Boman, Dr Emmanuelle Charafe-Jauffret, Dr Gabriela Dontu, Dr Suling Liu and Dr Christophe Ginestier for their advice and critical review of this manuscript. The HER2 construct is a generous gift from Dr Ignatoski. This work was supported by NIH Grants CA129765 and CA101860 and in part by the University of Michigan Cancer Center NIH support Grant 5 P 30 CA46592 and by the Taubman Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Korkaya, H., Paulson, A., Iovino, F. et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 27, 6120–6130 (2008). https://doi.org/10.1038/onc.2008.207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.207
Keywords
This article is cited by
-
Potential Role of Nrf2, HER2, and ALDH in Cancer Stem Cells: A Narrative Review
The Journal of Membrane Biology (2024)
-
Incidence and risk of fatal adverse events in cancer patients treated with HER2-targeted antibody-drug conjugates: a systematic review and meta-analysis of randomized controlled trials
BMC Cancer (2023)
-
Upregulation of SCD1 by ErbB2 via LDHA promotes breast cancer cell migration and invasion
Medical Oncology (2022)
-
Use of constitutive and inducible oncogene-containing iPSCs as surrogates for transgenic mice to study breast oncogenesis
Stem Cell Research & Therapy (2021)
-
Signaling pathways governing breast cancer stem cells behavior
Stem Cell Research & Therapy (2021)