Abstract
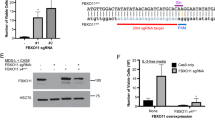
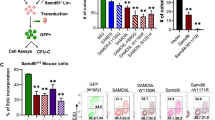
The myelodysplastic syndromes (MDSs) comprise a group of disorders characterized by multistage progression from cytopenias to acute myeloid leukemia (AML). They display exaggerated apoptosis in early stages, but lose this behavior during evolution to AML. The molecular basis for loss of apoptosis is unknown. To investigate this critical event, we analyzed phosphatidylinositol (PI) 3′kinase signaling, implicated as a critical pathway of cell survival control in epithelial and hematological malignancies. PI 3′kinase activates Akt through its production of 3′ phosphoinositides. In turn, the phosphoinositides are dephosphorylated by two lipid phosphatases, PTEN and SHIP-1, in myeloid cells. We studied primary MDS-enriched bone marrow cells and bone marrow sections by western blotting, immunohistochemistry, immunocytochemistry and quantitative PCR for components of the SHIP/PTEN/PI 3′kinase signaling circuit. We reported constitutively activated Akt, variable levels of PTEN and uniformly decreased SHIP-1 expression in MDS progenitor cells. Overexpression of SHIP-1, but not the phosphatase-deficient form, inhibited myeloid leukemic growth. Levels of microRNA (miR)-210 and miR-155 transcripts, which target SHIP-1, were increased in CD34+ MDS cells compared with their normal counterparts. Direct binding of miR-210 to the 3′ untranslated region of SHIP-1 was confirmed by luciferase reporter assay. Transfection of a myeloid cell line with miR-210 resulted in loss of SHIP-1 protein expression. These data suggest that miR-155 and miR-210/SHIP-1/Akt pathways could serve as clinical biomarkers for disease progression, and that miR-155 and miR-210 might serve as novel therapeutic targets in MDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aul C, Germing U, Gattermann N, Minning H . (1998). Increasing incidence of myelodysplastic syndromes: real or fictitious? Leuk Res 22: 93–100.
Cantley LC, Neel BG . (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA 96: 4240–4245.
Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD . (2007). Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer 7: 118–129.
Costinean S, Sandhu SK, Pedersen IM, Tili E, Trotta R, Perrotti D et al. (2009). Src homology 2 domain-containing inositol-5-phosphatase and CCAAT enhancer-binding protein beta are targeted by miR-155 in B cells of Emicro-MiR-155 transgenic mice. Blood 114: 1374–1382.
Dahia PL, Aguiar RC, Alberta J, Kum JB, Caron S, Sill H et al. (1999). PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum Mol Genet 8: 185–193.
Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW et al. (1996). The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA 93: 1689–1693.
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP . (1998). Pten is essential for embryonic development and tumour suppression. Nat Genet 19: 348–355.
Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK et al. (1998). Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science 280: 1069–1072.
Fenaux P . (2004). Myelodysplastic syndromes: from pathogenesis and prognosis to treatment. Semin Hematol 41: 6–12.
Follo MY, Mongiorgi S, Bosi C, Cappellini A, Finelli C, Chiarini F et al. (2007). The Akt/mammalian target of rapamycin signal transduction pathway is activated in high-risk myelodysplastic syndromes and influences cell survival and proliferation. Cancer Res 67: 4287–4294.
Geier SJ, Algate PA, Carlberg K, Flowers D, Friedman C, Trask B et al. (1997). The human SHIP gene is differentially expressed in cell lineages of the bone marrow and blood. Blood 89: 1876–1885.
Gilby DC, Goodeve AC, Winship PR, Valk PJ, Delwel R, Reilly JT . (2007). Gene structure, expression profiling and mutation analysis of the tumour suppressor SHIP1 in Caucasian acute myeloid leukaemia. Leukemia 21: 2390–2393.
Greenberg PL . (1998). Apoptosis and its role in the myelodysplastic syndromes: implications for disease natural history and treatment. Leuk Res 22: 1123–1136.
Greenberg PL, Young NS, Gattermann N . (2002). Myelodysplastic syndromes. Hematology (Am Soc Hematol Educ Program), 2002: 136–161.
Hazen AL, Smith MJ, Desponts C, Winter O, Moser K, Kerr WG . (2009). SHIP is required for a functional hematopoietic stem cell niche. Blood 113: 2924–2933.
Heaney ML, Golde DW . (1999). Myelodysplasia. N Engl J Med 340: 1649–1660.
Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM et al. (1998). Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev 12: 1610–1620.
Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK et al. (2009). Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35: 856–867.
Kalesnikoff J, Sly LM, Hughes MR, Buchse T, Rauh MJ, Cao LP et al. (2003). The role of SHIP in cytokine-induced signaling. Rev Physiol Biochem Pharmacol 149: 87–103.
Krystal G . (2000). Lipid phosphatases in the immune system. Semin Immunol 12: 397–403.
Lakhanpal GK, Vecchiarelli-Federico LM, Li YJ, Cui JW, Bailey ML, Spaner DE et al. (2010). The inositol phosphatase SHIP-1 is negatively regulated by Fli-1 and its loss accelerates leukemogenesis. Blood 116: 428–436.
Luo JM, Liu ZL, Hao HL, Wang FX, Dong ZR, Ohno R . (2004). Mutation analysis of SHIP gene in acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 12: 420–426.
Manning BD, Cantley LC . (2007). AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274.
Matsuda M, Morita Y, Hanamoto H, Tatsumi Y, Maeda Y, Kanamaru A . (2004). CD34+ progenitors from MDS patients are unresponsive to SDF-1, despite high levels of SDF-1 in bone marrow plasma. Leukemia 18: 1038–1040.
Melcher M, Unger B, Schmidt U, Rajantie IA, Alitalo K, Ellmeier W . (2008). Essential roles for the Tec family kinases Tec and Btk in M-CSF receptor signaling pathways that regulate macrophage survival. J Immunol 180: 8048–8056.
Moody JL, Xu L, Helgason CD, Jirik FR . (2004). Anemia, thrombocytopenia, leukocytosis, extramedullary hematopoiesis, and impaired progenitor function in Pten+/−SHIP−/− mice: a novel model of myelodysplasia. Blood 103: 4503–4510.
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA et al. (2004). PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6: 117–127.
Nyakern M, Tazzari PL, Finelli C, Bosi C, Follo MY, Grafone T et al. (2006). Frequent elevation of Akt kinase phosphorylation in blood marrow and peripheral blood mononuclear cells from high-risk myelodysplastic syndrome patients. Leukemia 20: 230–238.
O'Connell RM, Chaudhuri AA, Rao DS, Baltimore D . (2009). Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA 106: 7113–7118.
Pedersen IM, Otero D, Kao E, Miletic AV, Hother C, Ralfkiaer E et al. (2009). Onco-miR-155 targets SHIP1 to promote TNFalpha-dependent growth of B cell lymphomas. EMBO Mol Med 1: 288–295.
Pons A, Nomdedeu B, Navarro A, Gaya A, Gel B, Diaz T et al. (2009). Hematopoiesis-related microRNA expression in myelodysplastic syndromes. Leuk Lymphoma 50: 1854–1859.
Robertson GP, Furnari FB, Miele ME, Glendening MJ, Welch DR, Fountain JW et al. (1998). In vitro loss of heterozygosity targets the PTEN/MMAC1 gene in melanoma. Proc Natl Acad Sci USA 95: 9418–9423.
Rosenfeld C, List A . (2000). A hypothesis for the pathogenesis of myelodysplastic syndromes: implications for new therapies. Leukemia 14: 2–8.
Ruifrok AC, Johnston DA . (2001). Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291–299.
Ruschmann J, Ho V, Antignano F, Kuroda E, Lam V, Ibaraki M et al. (2010). Tyrosine phosphorylation of SHIP promotes its proteasomal degradation. Exp Hematol 38: 392–402, 402.e391.
Sato K, Mano H, Ariyama T, Inazawa J, Yazaki Y, Hirai H . (1994). Molecular cloning and analysis of the human Tec protein-tyrosine kinase. Leukemia 8: 1663–1672.
Sattler M, Verma S, Byrne CH, Shrikhande G, Winkler T, Algate PA et al. (1999). BCR/ABL directly inhibits expression of SHIP, an SH2-containing polyinositol-5-phosphatase involved in the regulation of hematopoiesis. Mol Cell Biol 19: 7473–7480.
Thompson JE, Conlon JP, Yang X, Sanchez PV, Carroll M . (2007). Enhanced growth of myelodysplastic colonies in hypoxic conditions. Exp Hematol 35: 21–31.
Tomlinson MG, Heath VL, Turck CW, Watson SP, Weiss A . (2004). SHIP family inositol phosphatases interact with and negatively regulate the Tec tyrosine kinase. J Biol Chem 279: 55089–55096.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. (2009). The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114: 937–951.
Ware MD, Rosten P, Damen JE, Liu L, Humphries RK, Krystal G . (1996). Cloning and characterization of human SHIP, the 145-kD inositol 5-phosphatase that associates with SHC after cytokine stimulation. Blood 88: 2833–2840.
Whichard ZL, Motter AE, Stein PJ, Corey SJ . (2011). Slowly produced MicroRNAs control protein levels. J Biol Chem 286: 4742–4748.
Worm J, Stenvang J, Petri A, Frederiksen KE, Obad S, Elmen J et al. (2009). Silencing of microRNA-155 in mice during inflammatory response leads to derepression of c/ebpa beta and down-regulation of G-CSF. Nucl Acids Res 37: 5784–5792.
Yamanaka Y, Tagawa H, Takahashi N, Watanabe A, Guo YM, Iwamoto K et al. (2009). Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood 114: 3265–3275.
Zysman MA, Chapman WB, Bapat B . (2002). Considerations when analyzing the methylation status of PTEN tumor suppressor gene. Am J Pathol 160: 795–800.
Acknowledgements
We thank Martin H Nguyen for technical assistance. We thank Drs Gerald Krystal for providing the cDNA for SHIP-1 and Maria-Magdalena Georgescu for control lysates. SJC was funded by the NIH Independent Scientist Award KO2-HL03794, RO1-CA108992, American Cancer Society Research Scholar Grant DHP-135, Ladies Leukemia League, JP McCarthy Foundation, NIH PO1CA55164 and Leukemia SPORE CA100632. Funding also came from the AA/MDS International Foundation to both SJC and MF.
Author contributions: MC, CEB-R, DWL and SJC designed the research. DWL, YF, ZW, MF, ZW and SAJ performed the research.SK and EJS contributed the reagents.DWL, MF, MC, CEB-R, ZW and SJC analyzed the data. DWL, MF, ZW and SJC wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, D., Futami, M., Carroll, M. et al. Loss of SHIP-1 protein expression in high-risk myelodysplastic syndromes is associated with miR-210 and miR-155. Oncogene 31, 4085–4094 (2012). https://doi.org/10.1038/onc.2011.579
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.579
Keywords
This article is cited by
-
MicroRNA-155 in serum-derived extracellular vesicles as a potential biomarker for hematologic malignancies - a short report
Cellular Oncology (2017)
-
miR-139-5p controls translation in myeloid leukemia through EIF4G2
Oncogene (2016)
-
Coordinate regulation of residual bone marrow function by paracrine trafficking of AML exosomes
Leukemia (2015)
-
MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis
Arthritis Research & Therapy (2014)
-
Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia
Journal of Hematology & Oncology (2013)