Abstract
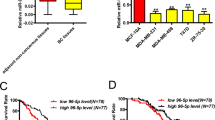
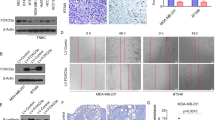
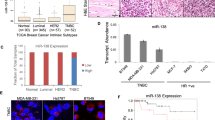
The role of TGF-β signaling in tumorigenesis is paradoxical: it can be tumor suppressive or tumor promotional, depending on context. The metastatic regulator, Six1, was recently shown to mediate this switch, providing a novel means to explain this elusive ‘TGF-β paradox’. Herein, we identify a mechanism by which Six1 activates the tumor promotional arm of TGF-β signaling, via its ability to upregulate the miR-106b-25 microRNA cluster, and further identify a novel function for this cluster of microRNAs. Although expression of the miR-106b-25 cluster is known to overcome TGF-β-mediated growth suppression via targeting p21 and BIM, we demonstrate for the first time that this same cluster can additionally target the inhibitory Smad7 protein, resulting in increased levels of the TGF-β type I receptor and downstream activation of TGF-β signaling. We further show that the miR-106b-25 cluster is sufficient to induce an epithelial-to-mesenchymal transition and a tumor initiating cell phenotype, and that it is required downstream of Six1 to induce these phenotypes. Finally, we demonstrate a significant correlation between miR-106b, Six1, and activated TGF-β signaling in human breast cancers, and further show that high levels of miR-106b and miR-93 in breast tumors significantly predicts shortened time to relapse. These findings expand the spectrum of oncogenic functions of miR-106b-25, and may provide a novel molecular explanation, through the Six1 regulated miR-106b-25 cluster, by which TGF-β signaling shifts from tumor suppressive to tumor promoting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Massagué J . TGFbeta in Cancer. Cell 2008; 134: 215–230.
Inman GJ . Switching TGFβ from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev 2011; 21: 93–99.
Tian M, Schiemann WP . Future Oncol 2009; 5: 259–271.
Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB . Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci USA 1998; 95: 12608–12613.
Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL . Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res 2008; 68: 2204–2213.
Reichenberger KJ, Coletta RD, Schulte AP, Varella-Garcia M, Ford HL . Gene amplification is a mechanism of Six1 overexpression in breast cancer. Cancer Res 2005; 65: 2668–2675.
Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Barón AE, Harrell JC et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-β signaling. J Clin Invest 2009; 119: 2678–2690.
McCoy EL, Iwanaga R, Jedlicka P, Abbey NS, Chodosh LA, Heichman KA et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest 2009; 119: 2663–2677.
Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL . Homeoprotein Six1 Increases TGF- Type I Receptor and Converts TGF- Signaling from Suppressive to Supportive for Tumor Growth. Cancer Res 2010; 70: 10371–10380.
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S et al. A microRNA polycistron as a potential human oncogene. Nature 2005; 435: 828–833.
Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I et al. E2F1-Regulated MicroRNAs Impair TGFβ-Dependent Cell-Cycle Arrest and Apoptosis in Gastric Cancer. Cancer Cell 2008; 13: 272–286.
Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG et al. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci 2009; 100: 1234–1242.
Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology 2009; 136: 1689–1700.
Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A . The microRNA cluster miR-106b∼25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging (Albany NY) 2011; 3: 108–124.
Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM et al. Identification of the miR-106b∼25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal 2010; 3: ra29.
Chopra VS, Mishra RK . ‘Mir’acles in hox gene regulation. Bioessays 2006; 28: 445–448.
Hu Y-L, Fong S, Largman C, Shen W-F . HOXA9 regulates miR-155 in hematopoietic cells, 2010; 38: 5472–5478.
Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene 2011; 30: 806–821.
Yan X, Liu Z, Chen Y . Regulation of TGF-β signaling by Smad7. Acta Biochimica et Biophysica Sinica 2009; 41: 263–272.
Yan X, Chen YG . Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J 2011; 434: 1–10.
Li Z, Yang C-S, Nakashima K, Rana TM . Small RNA-mediated regulation of iPS cell generation. EMBO J 2011; 30: 823–834.
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR et al. TGFβ Primes Breast Tumors for Lung Metastasis Seeding through Angiopoietin-like 4. Cell 2008; 133: 66–77.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Farabaugh SM, Micalizzi DS, Jedlicka P, Zhao R, Ford HL . Eya2 is required to mediate the pro-metastatic functions of Six1 via the induction of TGF-β signaling, epithelial-mesenchymal transition, and cancer stem cell properties. Oncogene 2012; 31: 552–562.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002; 347: 1999–2009.
Buffa FM, Camps C, Winchester L, Snell CE, Gee HE, Sheldon H et al. microRNA-Associated Progression Pathways and Potential Therapeutic Targets Identified by Integrated mRNA and microRNA Expression Profiling in Breast Cancer. Cancer Res 2011; 71: 5635–5645.
Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY . Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2008; 2: 333–344.
Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J . Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 2008; 68: 989–997.
Qian S, Ding JY, Xie R, An JH, Ao XJ, Zhao ZG et al. MicroRNA expression profile of bronchioalveolar stem cells from mouse lung. Biochem Biophys Res Commun 2008; 377: 668–673.
Ho J, Pandey P, Schatton T, Sims-Lucas S, Khalid M, Frank MH et al. The pro-apoptotic protein Bim is a microRNA target in kidney progenitors. J Am Soc Nephrol 2011; 22: 1053–1063.
Kakarala M, Wicha MS . Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 2008; 26: 2813–2820.
Nana-Sinkam SP, Croce CM . MicroRNAs as therapeutic targets in cancer, 2011; 157: 216–225.
Ford HL, Landesman-Bollag E, Dacwag CS, Stukenberg PT, Pardee AB, Seldin DC . Cell cycle-regulated phosphorylation of the human SIX1 homeodomain protein. J Biol Chem 2000; 275: 22245–22254.
Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB . Cell Adhesion Molecule L1 Disrupts E-Cadherin-Containing Adherens Junctions and Increases Scattering and Motility of MCF7 Breast Carcinoma Cells. Cancer Res 2006; 66: 11370–11380.
Jørgensen S, Baker A, Møller S, Nielsen BS . Robust one-day in situ hybridization protocol for detection of microRNAs in paraffin samples using LNA probes. Methods 2010; 52: 375–381.
Acknowledgements
The authors acknowledge the strong support of the University of Colorado Cancer Center for use of their core facilities (Flow Cytometry Core and DNA Sequencing and Analysis Core), as well as to the Genomics and Microarray Core at the University of Colorado. We would also like to thank Dr Paul Jedlicka for input and critical reading of the manuscript.
Financial Support: This work was funded by grants from the National Cancer Institute (2ROI-CA095277) and The American Cancer Society (no. RSG-07-183-01-DDC) to HLF. ALS is funded by a predoctoral fellowship from the Department of Defense Breast Cancer Research Program (W81XWH-10-1-0296). DSM was funded by a predoctoral fellowship from the Department of Defense Breast Cancer Research Program (W81XWH-06-1-0757).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Smith, A., Iwanaga, R., Drasin, D. et al. The miR-106b-25 cluster targets Smad7, activates TGF-β signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene 31, 5162–5171 (2012). https://doi.org/10.1038/onc.2012.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.11
Keywords
This article is cited by
-
MicroRNA-338-3p helps regulate ovarian function by affecting granulosa cell function and early follicular development
Journal of Ovarian Research (2023)
-
miR-106b enhances human mesenchymal stem cell differentiation to spermatogonial stem cells under germ cell profile genes involved in TGF-b signaling pathways
In Vitro Cellular & Developmental Biology - Animal (2022)
-
Synergistic Beneficial Effect of Docosahexaenoic Acid (DHA) and Docetaxel on the Expression Level of Matrix Metalloproteinase-2 (MMP-2) and MicroRNA-106b in Gastric Cancer
Journal of Gastrointestinal Cancer (2020)
-
MicroRNAs, a Promising Target for Breast Cancer Stem Cells
Molecular Diagnosis & Therapy (2020)
-
Multivariate analysis of clinicopathological and prognostic significance of miRNA 106b~25 cluster in gastric cancer
Cancer Cell International (2019)