Abstract
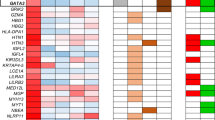
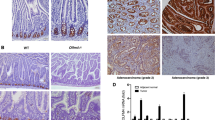
Cancer arises as the consequence of mutations and epigenetic alterations that activate oncogenes and inactivate tumor suppressor genes. Through a genome-wide screen for methylated genes in colon neoplasms, we identified aberrantly methylated RET in colorectal cancer. RET, a transmembrane receptor tyrosine kinase and a receptor for the glial cell-derived neurotrophic factor family ligands, was one of the first oncogenes to be identified, and has been shown to be an oncogene in thyroid cancer and pheochromocytoma. However, unexpectedly, we found RET is methylated in 27% of colon adenomas and in 63% of colorectal cancers, and now provide evidence that RET has tumor suppressor activity in colon cancer. The aberrant methylation of RET correlates with decreased RET expression, whereas the restoration of RET in colorectal cancer cell lines results in apoptosis. Furthermore, in support of a tumor suppressor function of RET, mutant RET has also been found in primary colorectal cancer. We now show that these mutations inactivate RET, which is consistent with RET being a tumor suppressor gene in the colon. These findings suggest that the aberrant methylation of RET and the mutational inactivation of RET promote colorectal cancer formation, and that RET can serve as a tumor suppressor gene in the colon. Moreover, the increased frequency of methylated RET in colon cancers compared with adenomas suggests RET inactivation is involved in the progression of colon adenomas to cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Kondo Y, Issa JP . Epigenetic changes in colorectal cancer. Cancer Metastasis Rev 2004; 23: 29–39.
Estecio MR, Yan PS, Ibrahim AE, Tellez CS, Shen L, Huang TH et al. High-throughput methylation profiling by MCA coupled to CpG island microarray. Genome Res 2007; 17: 1529–1536.
Chan TA, Glockner S, Yi JM, Chen W, Van Neste L, Cope L et al. Convergence of mutation and epigenetic alterations identifies common genes in cancer that predict for poor prognosis. PLoS Med 2008; 5: e114.
Hinoue T, Weisenberger DJ, Lange CP, Shen H, Byun HM, Van Den Berg D et al. Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Res 2012; 22: 271–282.
Rojas A, Meherem S, Kim YH, Washington MK, Willis JE, Markowitz SD et al. The aberrant methylation of TSP1 suppresses TGF-beta1 activation in colorectal cancer. Int J Cancer 2008; 123: 14–21.
Veigl M, Kasturi L, Olechnowicz J, Ma A, Lutterbaugh J, Periyasamy S et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci USA 1998; 95: 8698–8702.
Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 1995; 55: 4525–4530.
Bozic I, Antal T, Ohtsuki H, Carter H, Kim D, Chen S et al. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci USA 2010; 107: 18545–18550.
Issa JP . CpG island methylator phenotype in cancer. Nat Rev Cancer 2004; 4: 988–993.
Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006; 314: 268–274.
Alhopuro P, Sammalkorpi H, Niittymaki I, Bistrom M, Raitila A, Saharinen J et al. Candidate driver genes in microsatellite-unstable colorectal cancer. Int J Cancer 2012; 130: 1558–1566.
Eskiocak U, Kim SB, Ly P, Roig AI, Biglione S, Komurov K et al. Functional parsing of driver mutations in the colorectal cancer genome reveals numerous suppressors of anchorage-independent growth. Cancer Res 2011; 71: 4359–4365.
Wells SA, Santoro M . Targeting the RET pathway in thyroid cancer. Clin Cancer Res 2009; 15: 7119–7123.
Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar A-R et al. Effect of conditional knockout of the type II TGFB receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res 2005; 65: 2296–2302.
Muraoka-Cook RS, Shin I, Yi JY, Easterly E, Barcellos-Hoff MH, Yingling JM et al. Activated type I TGFbeta receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene 2006; 25: 3408–3423.
Bierie B, Moses HL . Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 2006; 6: 506–520.
Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K . Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell 2009; 5: 504–514.
Grady W, Rajput A, Myeroff L, Liu D, Willis J, Kwon K et al. Mutation of the type II TGF-β receptor is coincident with the transformation of human colon adenomas to malignant carcinomas. Cancer Res 1998; 58: 3101–3104.
Jain S, Knoten A, Hoshi M, Wang H, Vohra B, Heuckeroth RO et al. Organotypic specificity of key RET adaptor-docking sites in the pathogenesis of neurocristopathies and renal malformations in mice. J Clin Invest 2010; 120: 778–77 790.
Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V . Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994; 367: 380–383.
Popsueva A, Poteryaev D, Arighi E, Meng X, Angers-Loustau A, Kaplan D et al. GDNF promotes tubulogenesis of GFRalpha1-expressing MDCK cells by Src-mediated phosphorylation of Met receptor tyrosine kinase. J Cell Biol 2003; 161: 119–129.
Wartiovaara K, Salo M, Sainio K, Rintala R, Sariola H . Distribution of glial cell line-derived neurotrophic factor mRNA in human colon suggests roles for muscularis mucosae in innervation. J Pediatr Surg 1998; 33: 1501–1506.
Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature 1996; 381: 789–793.
Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T et al. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J Biol Chem 1997; 272: 33111–33117.
Fukuda T, Kiuchi K, Takahashi M . Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J Biol Chem 2002; 277: 19114–19121.
Airaksinen MS, Saarma M . The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002; 3: 383–394.
Borrello MG, Alberti L, Arighi E, Bongarzone I, Battistini C, Bardelli A et al. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol Cell Biol 1996; 16: 2151–2163.
Bogen O, Joseph EK, Chen X, Levine JD . GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci 2008; 28: 12–19.
Diaz-Rodriguez E, Garcia-Lavandeira M, Perez-Romero S, Senra A, Canibano C, Palmero I et al. Direct promoter induction of p19Arf by Pit-1 explains the dependence receptor RET/Pit-1/p53-induced apoptosis in the pituitary somatotroph cells. Oncogene 2012; 31: 2824–2835.
Goldschneider D, Mehlen P . Dependence receptors: a new paradigm in cell signaling and cancer therapy. Oncogene 2010; 29: 1865–1882.
Trupp M, Scott R, Whittemore SR, Ibanez CF . Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem 1999; 274: 20885–20894.
Arighi E, Borrello MG, Sariola H . RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 2005; 16: 441–467.
Pasini B, Borrello MG, Greco A, Bongarzone I, Luo Y, Mondellini P et al. Loss of function effect of RET mutations causing Hirschsprung disease. Nat Genet 1995; 10: 35–40.
Asai N, Jijiwa M, Enomoto A, Kawai K, Maeda K, Ichiahara M et al. RET receptor signaling: dysfunction in thyroid cancer and Hirschsprung’s disease. Pathol Int 2006; 56: 164–172.
Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M . Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 1996; 382: 70–73.
Shen L, Kondo Y, Guo Y, Zhang J, Zhang L, Ahmed S et al. Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet 2007; 3: 2023–2036.
Eads CA, Lord RV, Kurumboor SK, Wickramasinghe K, Skinner ML, Long TI et al. Fields of aberrant CpG island hypermethylation in Barrett’s esophagus and associated adenocarcinoma. Cancer Res 2000; 60: 5021–5026.
Ahuja N, Li Q, Mohan M, Baylin S, Issa J-P . Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res 1998; 58: 5489–5494.
Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res 2001; 61: 3410–3418.
Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006; 38: 787–793.
Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG et al. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 1998; 20: 245–253.
Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M et al. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 2000; 19: 4056–4063.
Rojas A, Padidam M, Cress D, Grady WM . TGF-β receptor levels regulated the specificity of signaling pathway activation and biological effects of TGF-β. Biochim Biophys Acta-Mol Cell Res 2009; 1793: 1165–1173.
Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 2006; 131: 1096–1109.
Sipos JA, Shah MH . Thyroid cancer: emerging role for targeted therapies. Ther Adv Med Oncol 2: 3–16.
Arslan-Kirchner M, Epplen JT, Faivre L, Jondeau G, Schmidtke J, De Paepe A et al. Clinical utility gene card for: Loeys-Dietz syndrome (TGFBR1/2) and related phenotypes. Eur J Hum Genet 2011; 19: 1108.
Bernet A, Mazelin L, Coissieux MM, Gadot N, Ackerman SL, Scoazec JY et al. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology 2007; 133: 1840–1848.
Mehlen P, Fearon ER . Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol 2004; 22: 3420–3428.
Shin SK, Nagasaka T, Jung BH, Matsubara N, Kim WH, Carethers JM et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology 2007; 133: 1849–1857.
Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA 2005; 102: 15785–15790.
Kang J, Perry JK, Pandey V, Fielder GC, Mei B, Qian PX et al. Artemin is oncogenic for human mammary carcinoma cells. Oncogene 2009; 28: 2034–2045.
Tang JZ, Kong XJ, Kang J, Fielder GC, Steiner M, Perry JK et al. Artemin-stimulated progression of human non-small cell lung carcinoma is mediated by BCL2. Mol Cancer Ther 2010; 9: 1697–1708.
Grady WM, Parkin RK, Mitchell PS, Lee JH, Kim YH, Tsuchiya KD et al. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene 2008; 27: 3880–3888.
Markowitz S, Myeroff L, Cooper M, Traicoff J, Kochera M, Lutterbaugh J et al. A benign cultured colon adenoma bears three genetically altered colon cancer oncogenes, but progresses to tumorigenicity and transforming growth factor-beta independence without inactivating the p53 tumor suppressor gene. J Clin Invest 1994; 93: 1005–1013.
Williams A, Harper S, Paraskeva C . Neoplastic transformation of a human colonic epithelial cell line: in vitro evidence for the adenoma to carcinoma sequence. Cancer Res 1990; 50: 4724–4730.
Willson J, Bittner G, Oberley T, Meisner G, Weese J . Cell culture of human colon adenomas and carcinomas. Cancer Res 1987; 47: 2704–2713.
Paraskeva C, Finerty S, Mountford R, Powell S . Specific cytogenetic abnormalities in two new human colorectal adenoma-derived epithelial cell lines. Cancer Res 1989; 49: 1282–1286.
Gan FF, Nagle AA, Ang X, Ho OH, Tan SH, Yang H et al. Shogaols at proapoptotic concentrations induce G(2)/M arrest and aberrant mitotic cell death associated with tubulin aggregation. Apoptosis 2011; 16: 856–867.
Acknowledgements
Support for these studies was provided by the NIH (RO1CA115513, P30CA15704, U54CA143862, UO1CA152756, WMG), and a Burroughs Wellcome Fund Translational Research Award for Clinician Scientist (WMG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Luo, Y., Tsuchiya, K., Il Park, D. et al. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene 32, 2037–2047 (2013). https://doi.org/10.1038/onc.2012.225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.225
Keywords
This article is cited by
-
Epigenetic silencing and tumor suppressor gene of HAND2 by targeting ERK signaling in colorectal cancer
Cell Communication and Signaling (2022)
-
Modular and mechanistic changes across stages of colorectal cancer
BMC Cancer (2022)
-
OLFM4-RET fusion is an oncogenic driver in small intestine adenocarcinoma
Oncogene (2022)
-
Comprehensive profiling of 1015 patients’ exomes reveals genomic-clinical associations in colorectal cancer
Nature Communications (2022)
-
Genome-wide analysis identifies critical DNA methylations within NTRKs genes in colorectal cancer
Journal of Translational Medicine (2021)