Abstract
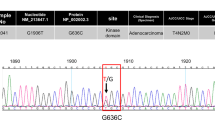
Grb2-associated binder 1 (Gab1) is a docking protein that transduces signals from a variety of tyrosine kinases, including Met and the epidermal growth factor receptor (EGFR). Although the related protein Gab2 is strongly implicated in human cancer, a role for Gab1 has been less clear. However, a screen for gene mutations in breast cancer identified two somatic mutations in Gab1, Y83C and T387N. In this paper we describe the functional characterization of these Gab1 mutants. MCF-10A immortalized mammary epithelial cells overexpressing Gab1 Y83C and T387N exhibited a more elongated, fibroblastic phenotype compared with wild-type Gab1 controls. Expression of Gab1 or the mutants promoted epidermal growth factor (EGF)-independent proliferation in monolayer culture to a similar degree. However, in Matrigel culture, both mutants enhanced the formation of acini exhibiting an aberrant, branched morphology. In addition, expression of the mutants modestly increased Erk activation. The two mutants also enhanced branching morphogenesis in a different mammary epithelial cell line, HC11. To gain further insights into the mechanism of action of these mutations, we mapped Gab1 phosphorylation sites by mass spectrometry. This detected phosphorylation of T387 but ;not Y83. Cellular stimulation with EGF or hepatocyte growth factor (HGF) led to a transient, or sustained, induction of T387 phosphorylation, respectively. As T387 corresponds in position to Gab2 T391, which suppresses Gab2 signaling in a phosphorylation-dependent manner, these data support a model in which the T387N mutation abrogates negative-feedback regulation of Gab1. Interrogation of publically-available databases revealed additional cancer-associated mutations at, or in close proximity to, identified serine/threonine phosphorylation sites in other docking proteins. These data indicate that aberrant Gab1 signaling can directly contribute to breast cancer progression, and that negative feedback sites in docking proteins can be targeted by oncogenic mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wohrle FU, Daly RJ, Brummer T . Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal 2009; 7: 22.
Lock LS, Frigault MM, Saucier C, Park M . Grb2-independent recruitment of Gab1 requires the C-terminal lobe and structural integrity of the Met receptor kinase domain. J Biol Chem 2003; 278: 30083–30090.
Lock LS, Royal I, Naujokas MA, Park M . Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem 2000; 275: 31536–31545.
Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J . A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol 2000; 20: 1448–1459.
Maroun CR, Holgado-Madruga M, Royal I, Naujokas MA, Fournier TM, Wong AJ et al. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol 1999; 19: 1784–1799.
Abella JV, Vaillancourt R, Frigault MM, Ponzo MG, Zuo D, Sangwan V et al. The Gab1 scaffold regulates RTK-dependent dorsal ruffle formation through the adaptor Nck. J Cell Sci 2010; 123: 1306–1319.
Mood K . Saucier C, Bong YS, Lee HS, Park M, Daar IO. Gab1 is required for cell cycle transition, cell proliferation, and transformation induced by an oncogenic met receptor. Mol Biol Cell 2006; 17: 3717–3728.
Laramee M, Chabot C, Cloutier M, Stenne R, Holgado-Madruga M, Wong AJ et al. The scaffolding adapter Gab1 mediates vascular endothelial growth factor signaling and is required for endothelial cell migration and capillary formation. J Biol Chem 2007; 282: 7758–7769.
Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M . The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol 2000; 20: 8513–8525.
Eulenfeld R, Schaper F . A new mechanism for the regulation of Gab1 recruitment to the plasma membrane. J Cell Sci 2009; 122: 55–64.
Yu CF, Roshan B, Liu ZX, Cantley LG . ERK regulates the hepatocyte growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J Biol Chem 2001; 276: 32552–32558.
Gual P, Giordano S, Anguissola S, Parker PJ, Comoglio PM . Gab1 phosphorylation: a novel mechanism for negative regulation of HGF receptor signaling. Oncogene 2001; 20: 156–166.
Lynch DK, Daly RJ . PKB-mediated negative feedback tightly regulates mitogenic signalling via Gab2. EMBO J 2002; 21: 72–82.
Brummer T, Larance M, Herrera Abreu MT, Lyons RJ, Timpson P, Emmerich CH et al. Phosphorylation-dependent binding of 14-3-3 terminates signalling by the Gab2 docking protein. EMBO J 2008; 27: 2305–2316.
Arnaud M, Crouin C, Deon C, Loyaux D, Bertoglio J . Phosphorylation of Grb2-associated binder 2 on serine 623 by ERK MAPK regulates its association with the phosphatase SHP-2 and decreases STAT5 activation. J Immunol 2004; 173: 3962–3971.
Bentires-Alj M, Gil SG, Chan R, Wang ZC, Wang Y, Imanaka N et al. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med 2006; 12: 114–121.
Bocanegra M, Bergamaschi A, Kim YH, Miller MA, Rajput AB, Kao J et al. Focal amplification and oncogene dependency of GAB2 in breast cancer. Oncogene 2010; 29: 774–779.
Brown LA, Kalloger SE, Miller MA, Shih Ie M, McKinney SE, Santos JL et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer 2008; 47: 481–489.
Chernoff KA, Bordone L, Horst B, Simon K, Twadell W, Lee K et al. GAB2 amplifications refine molecular classification of melanoma. Clin Cancer Res 2009; 15: 4288–4291.
Daly RJ, Gu H, Parmar J, Malaney S, Lyons RJ, Kairouz R et al. The docking protein Gab2 is overexpressed and estrogen regulated in human breast cancer. Oncogene 2002; 21: 5175–5181.
Fleuren ED, O'Toole S, Millar EK, McNeil C, Lopez-Knowles E, Boulghourjian A et al. Overexpression of the oncogenic signal transducer Gab2 occurs early in breast cancer development. Int J Cancer 2010; 127: 1486–1492.
Horst B, Gruvberger-Saal SK, Hopkins BD, Bordone L, Yang Y, Chernoff KA et al. Gab2-mediated signaling promotes melanoma metastasis. Am J Pathol 2009; 174: 1524–1533.
Lee SH, Jeong EG, Nam SW, Lee JY, Yoo NJ . Increased expression of Gab2, a scaffolding adaptor of the tyrosine kinase signalling, in gastric carcinomas. Pathology 2007; 39: 326–329.
Zatkova A, Schoch C, Speleman F, Poppe B, Mannhalter C, Fonatsch C et al. GAB2 is a novel target of 11q amplification in AML/MDS. Genes Chromosomes Cancer 2006; 45: 798–807.
Juric D, Lacayo NJ, Ramsey MC, Racevskis J, Wiernik PH, Rowe JM et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J Clin Oncol 2007; 25: 1341–1349.
Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 2011; 121: 381–396.
Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006; 314: 268–274.
Soule HD, Maloney TM, Wolman SR, Peterson WD, Brenz R, McGrath CM et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 1990; 50: 6075–6086.
Drasin DJ, Robin TP, Ford HL . Breast cancer epithelial-to-mesenchymal transition: examining the functional consequences of plasticity. Breast Cancer Res 2011; 13: 226.
Debnath J, Muthuswamy SK, Brugge JS . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30: 256–268.
Wrobel CN, Debnath J, Lin E, Beausoleil S, Roussel MF, Brugge JS . Autocrine CSF-1R activation promotes Src-dependent disruption of mammary epithelial architecture. J Cell Biol 2004; 165: 263–273.
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS . ErbB2 but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 2001; 3: 785–792.
Brummer T, Schramek D, Hayes VM, Bennett HL, Caldon CE, Musgrove EA et al. Increased proliferation and altered growth factor dependence of human mammary epithelial cells overexpressing the Gab2 docking protein. J Biol Chem 2006; 281: 626–637.
Reginato MJ, Mills KR, Becker EB, Lynch DK, Bonni A, Muthuswamy SK et al. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol Cell Biol 2005; 25: 4591–4601.
Khwaja A, Lehmann K, Marte BM, Downward J . Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem 1998; 273: 18793–18801.
Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B . Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J 1988; 7: 2089–2095.
Xian W, Schwertfeger KL, Vargo-Gogola T, Rosen JM . Pleiotropic effects of FGFR1 on cell proliferation, survival, and migration in a 3D mammary epithelial cell model. J Cell Biol 2005; 171: 663–673.
Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007; 448: 439–444.
Landgraf KE, Pilling C, Falke JJ . Molecular mechanism of an oncogenic mutation that alters membrane targeting: Glu17Lys modifies the PIP lipid specificity of the AKT1 PH domain. Biochemistry 2008; 47: 12260–12269.
Ferguson KM, Kavran JM, Sankaran VG, Fournier E, Isakoff SJ, Skolnik EY et al. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol Cell 2000; 6: 373–384.
Aitken A . 14-3-3 proteins: a historic overview. Semin Cancer Biol 2006; 16: 162–172.
Janes PW, Daly RJ, deFazio A, Sutherland RL . Activation of the Ras signalling pathway in human breast cancer cells overexpressing erbB-2. Oncogene 1994; 9: 3601–3608.
Acknowledgements
This work was supported by the National Health and Medical Research Council of Australia. CEC and DRC are recipients of Cancer Institute New South Wales Early Career Fellowships. DG-O is a National Breast Cancer Foundation and Cure Cancer Australia Fellow. CJO is supported by New South Wales Cancer Council and Banque Nationale de Paris-Paribas Australia. TB is supported by the German Research Foundation (DFG) through the Emmy-Noether-Program, CRC850 and EXC294 BIOSS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ortiz-Padilla, C., Gallego-Ortega, D., Browne, B. et al. Functional characterization of cancer-associated Gab1 mutations. Oncogene 32, 2696–2702 (2013). https://doi.org/10.1038/onc.2012.271
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.271
Keywords
This article is cited by
-
Dysregulated Gab1 signalling in triple negative breast cancer
Cell Communication and Signaling (2024)
-
BRAF inhibition upregulates a variety of receptor tyrosine kinases and their downstream effector Gab2 in colorectal cancer cell lines
Oncogene (2018)
-
Changes in Gab2 phosphorylation and interaction partners in response to interleukin (IL)-2 stimulation in T-lymphocytes
Scientific Reports (2016)
-
Scaffolding protein Gab1 regulates myeloid dendritic cell migration in allergic asthma
Cell Research (2016)
-
The dynamic control of signal transduction networks in cancer cells
Nature Reviews Cancer (2015)