Abstract
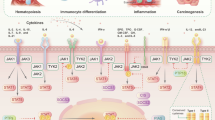
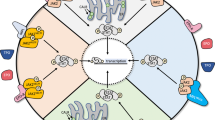
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is central to signaling by cytokine receptors, a superfamily of more than 30 transmembrane proteins that recognize specific cytokines, and is critical in blood formation and immune response. Many of those receptors transmit anti-apoptotic, proliferative and differentiation signals, and their expression and functions are critical for the formation of blood lineages. Several cancers, including blood malignancies, have been associated with constitutive activation of members of the STAT family, which normally require JAK-mediated tyrosine phosphorylation for transcriptional activation. More recently, human myeloproliferative neoplasms were discovered to be associated with a unique acquired somatic mutation in JAK2 (JAK2 V617F), rare exon 12 JAK2 mutations, or thrombopoietin receptor mutations that constitutively activate wild-type JAK2. Prompted by these observations, many studies have explored the possibility that JAKs, cytokine receptors, or other components of the JAK/STAT pathway are mutated or upregulated in several hematological malignancies. This has been observed in certain pediatric acute lymphoblastic leukemias and adult T-cell lymphoblastic leukemias, and overexpression of JAK2 seems to be important in Hodgkin lymphoma. Here we discuss the nature and respective contribution of mutations dysregulating the JAK/STAT pathway in hematological malignancies and present examples in which such mutations drive the disease, contribute to the phenotype, or provide a survival and proliferative advantage. JAK inhibitors are making their way into the therapeutic arsenal (for example, in myelofibrosis), and we discuss the possibility that other hematological diseases might benefit from treatment with these inhibitors in combination with other agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Velazquez L, Fellous M, Stark GR, Pellegrini S . A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell 1992; 70: 313–322.
Darnell JE, Kerr IM, Stark GR . Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264: 1415–1421.
Remy I, Wilson IA, Michnick SW . Erythropoietin receptor activation by a ligand-induced conformation change. Science 1999; 283: 990–993.
Constantinescu SN, Keren T, Socolovsky M, Nam H, Henis YI, Lodish HF . Ligand-independent oligomerization of cell-surface erythropoietin receptor is mediated by the transmembrane domain. Proc Natl Acad Sci USA 2001; 98: 4379–4384.
Constantinescu SN, Huang LJ, Nam H, Lodish HF . The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol Cell 2001; 7: 377–385.
Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K et al. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol 2005; 12: 814–821.
Thomas C, Moraga I, Levin D, Krutzik PO, Podoplelova Y, Trejo A et al. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell 2011; 146: 621–632.
Ihle JN . The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol 1995; 60: 1–35.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. Stat3 as an oncogene. Cell 1999; 98: 295–303.
Levy DE, Darnell JE . Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3: 651–662.
Migone TS, Lin JX, Cereseto A, Mulloy JC, O'Shea JJ, Franchini G et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 1995; 269: 79–81.
Weber-Nordt RM, Egen C, Wehinger J, Ludwig W, Gouilleux-Gruart V, Mertelsmann R et al. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood 1996; 88: 809–816.
Xia Z, Baer MR, Block AW, Baumann H, Wetzler M . Expression of signal transducers and activators of transcription proteins in acute myeloid leukemia blasts. Cancer Res 1998; 58: 3173–3180.
Buettner R, Mora LB, Jove R . Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 2002; 8: 945–954.
Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 1997; 278: 1309–1312.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365: 1054–1061.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434: 1144–1148.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352: 1779–1790.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005; 7: 387–397.
Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med 2008; 205: 751–758.
Tomasson MH, Xiang Z, Walgren R, Zhao Y, Kasai Y, Miner T et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood 2008; 111: 4797–4808.
Xiang Z, Zhao Y, Mitaksov V, Fremont DH, Kasai Y, Molitoris A et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood 2008; 111: 4809–4812.
Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA 2009; 106: 9414–9418.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006; 3: e270.
Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin MG, Liu W, Zhang J et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 2009; 41: 1243–1246.
Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, Calasanz MJ et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood 2009; 114: 2688–2698.
Zenatti PP, Ribeiro D, Li W, Zuurbier L, Silva MC, Paganin M et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat Genet 2011; 43: 932–939.
Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs KD et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood 2010; 116: 988–992.
Watowich SS, Wu H, Socolovsky M, Klingmuller U, Constantinescu SN, Lodish HF . Cytokine receptor signal transduction and the control of hematopoietic cell development. Annu Rev Cell Dev Biol 1996; 12: 91–128.
Huang LJ, Constantinescu SN, Lodish HF . The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol Cell 2001; 8: 1327–1338.
Radtke S, Hermanns HM, Haan C, Schmitz-Van De Leur H, Gascan H, Heinrich PC et al. Novel role of Janus kinase 1 in the regulation of oncostatin M receptor surface expression. J Biol Chem 2002; 277: 11297–11305.
Ragimbeau J, Dondi E, Alcover A, Eid P, Uze G, Pellegrini S . The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 2003; 22: 537–547.
Royer Y, Staerk J, Costuleanu M, Courtoy PJ, Constantinescu SN . Janus kinases affect thrombopoietin receptor cell surface localization and stability. J Biol Chem 2005; 280: 27251–27261.
Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature 1998; 395: 511–516.
Vainchenker W, Dusa A, Constantinescu SN . JAKs in pathology: role of Janus kinases in hematopoietic malignancies and immunodeficiencies. Semin Cell Dev Biol 2008; 19: 385–393.
Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 1993; 74: 227–236.
Drachman JG, Millett KM, Kaushansky K . Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem 1999; 274: 13480–13484.
Touw IP, van de Geijn GJ . Granulocyte colony-stimulating factor and its receptor in normal myeloid cell development, leukemia and related blood cell disorders. Front Biosci 2007; 12: 800–815.
Shimoda K, Feng J, Murakami H, Nagata S, Watling D, Rogers NC et al. Jak1 plays an essential role for receptor phosphorylation and Stat activation in response to granulocyte colony-stimulating factor. Blood 1997; 90: 597–604.
Sattler M, Durstin MA, Frank DA, Okuda K, Kaushansky K, Salgia R et al. The thrombopoietin receptor c-MPL activates JAK2 and TYK2 tyrosine kinases. Exp Hematol 1995; 23: 1040–1048.
Drachman JG, Kaushansky K . Dissecting the thrombopoietin receptor: functional elements of the Mpl cytoplasmic domain. Proc Natl Acad Sci USA 1997; 94: 2350–2355.
Constantinescu SN, Ghaffari S, Lodish HF . The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol Metab 1999; 10: 18–23.
van de Geijn GJ, Gits J, Aarts LH, Heijmans-Antonissen C, Touw IP . G-CSF receptor truncations found in SCN/AML relieve SOCS3-controlled inhibition of STAT5 but leave suppression of STAT3 intact. Blood 2004; 104: 667–674.
Liao W, Lin JX, Leonard WJ . IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol 2011; 23: 598–604.
Ghoreschi K, Laurence A, O'Shea JJ . Janus kinases in immune cell signaling. Immunol Rev 2009; 228: 273–287.
Haan C, Rolvering C, Raulf F, Kapp M, Druckes P, Thoma G et al. Jak1 has a dominant role over Jak3 in signal transduction through gammac-containing cytokine receptors. Chem Biol 2011; 18: 314–323.
Demoulin JB, Van Roost E, Stevens M, Groner B, Renauld JC . Distinct roles for STAT1, STAT3, and STAT5 in differentiation gene induction and apoptosis inhibition by interleukin-9. J Biol Chem 1999; 274: 25855–25861.
Verdier F, Walrafen P, Hubert N, Chretien S, Gisselbrecht S, Lacombe C et al. Proteasomes regulate the duration of erythropoietin receptor activation by controlling down-regulation of cell surface receptors. J Biol Chem 2000; 275: 18375–18381.
Gross AW, Lodish HF . Cellular trafficking and degradation of erythropoietin and novel erythropoiesis stimulating protein (NESP). J Biol Chem 2006; 281: 2024–2032.
Irandoust MI, Aarts LH, Roovers O, Gits J, Erkeland SJ, Touw IP . Suppressor of cytokine signaling 3 controls lysosomal routing of G-CSF receptor. EMBO J 2007; 26: 1782–1793.
Hitchcock IS, Chen MM, King JR, Kaushansky K . YRRLmotifs in the cytoplasmic domain of the thrombopoietin receptor regulate receptor internalization and degradation. Blood 2008; 112: 2222–2231.
Marijanovic Z, Ragimbeau J, Kumar KG, Fuchs SY, Pellegrini S . TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem J 2006; 397: 31–38.
Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF . Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 1995; 80: 729–738.
Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A . CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem 2000; 275: 29338–29347.
Wang Q, Miyakawa Y, Fox N, Kaushansky K . Interferon-alpha directly represses megakaryopoiesis by inhibiting thrombopoietin-induced signaling through induction of SOCS-1. Blood 2000; 96: 2093–2099.
Jegalian AG, Wu H . Differential roles of SOCS family members in EpoR signal transduction. J Interferon Cytokine Res 2002; 22: 853–860.
Hortner M, Nielsch U, Mayr LM, Heinrich PC, Haan S . A new high affinity binding site for suppressor of cytokine signaling-3 on the erythropoietin receptor. Eur J Biochem 2002; 269: 2516–2526.
Chaligne R, Tonetti C, Besancenot R, Marty C, Kiladjian JJ, Socie G et al. SOCS3 inhibits TPO-stimulated, but not spontaneous, megakaryocytic growth in primary myelofibrosis. Leukemia 2009; 23: 1186–1190.
Palvimo JJ . PIAS proteins as regulators of small ubiquitin-related modifier (SUMO) modifications and transcription. Biochem Soc Trans 2007; 35 (Pt 6): 1405–1408.
Zhou S, Si J, Liu T, DeWille JW . PIASy represses CCAAT/enhancer-binding protein delta (C/EBPdelta) transcriptional activity by sequestering C/EBPdelta to the nuclear periphery. J Biol Chem 2008; 283: 20137–20148.
Yoshimura A, Longmore G, Lodish HF . Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature 1990; 348: 647–649.
Alexander WS, Metcalf D, Dunn AR . Point mutations within a dimer interface homology domain of c-Mpl induce constitutive receptor activity and tumorigenicity. EMBO J 1995; 14: 5569–5578.
Longmore G, Watowich S, Pharr P, Neumann D, Lodish H . Activation of the erythropoietin receptor and leukemia induction in mice. Leukemia 1993; 7 (Suppl 2): S113–S116.
Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P et al. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood 1997; 90: 2535–2540.
Nguyen MH, Ho JM, Beattie BK, Barber DL . TEL-JAK2 mediates constitutive activation of the phosphatidylinositol 3'-kinase/protein kinase B signaling pathway. J Biol Chem 2001; 276: 32704–32713.
Schwaller J, Frantsve J, Aster J, Williams IR, Tomasson MH, Ross TS et al. Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myelo- and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion genes. EMBO J 1998; 17: 5321–5333.
Kennedy JA Barabe F, Patterson BJ, Bayani J, JA Squire, Barber DL et al. Expression of TEL-JAK2 in primary human hematopoietic cells drives erythropoietin-independent erythropoiesis and induces myelofibrosis in vivo. Proc Natl Acad Sci USA 2006; 103: 16930–16935.
Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res 2005; 65: 2662–2667.
Murati A, Gelsi-Boyer V, Adelaide J, Perot C, Talmant P, Giraudier S et al. PCM1-JAK2 fusion in myeloproliferative disorders and acute erythroid leukemia with t(8;9) translocation. Leukemia 2005; 19: 1692–1696.
Adelaide J, Perot C, Gelsi-Boyer V, Pautas C, Murati A, Copie-Bergman C et al. A t(8;9) translocation with PCM1-JAK2 fusion in a patient with T-cell lymphoma. Leukemia 2006; 20: 536–537.
Cirmena G, Aliano S, Fugazza G, Bruzzone R, Garuti A, Bocciardi R et al. A BCR-JAK2 fusion gene as the result of a t(9;22)(p24;q11) in a patient with acute myeloid leukemia. Cancer Genet Cytogenet 2008; 183: 105–108.
Poitras JL, Dal Cin P, Aster JC, Deangelo DJ, Morton CC . Novel SSBP2-JAK2 fusion gene resulting from a t(5;9)(q14.1;p24.1) in pre-B acute lymphocytic leukemia. Genes Chromosomes Cancer 2008; 47: 884–889.
Nebral K, Denk D, Attarbaschi A, Konig M, Mann G, Haas OA et al. Incidence and diversity of PAX5 fusion genes in childhood acute lymphoblastic leukemia. Leukemia 2009; 23: 134–143.
Van Roosbroeck K, Cox L, Tousseyn T, Lahortiga I, Gielen O, Cauwelier B et al. JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood 2011; 117: 4056–4064.
Knoops L, Hornakova T, Royer Y, Constantinescu SN, Renauld JC . JAK kinases overexpression promotes in vitro cell transformation. Oncogene 2008; 27: 1511–1519.
Navarro A, Diaz T, Martinez A, Gaya A, Pons A, Gel B et al. Regulation of JAK2 by miR-135a: prognostic impact in classic Hodgkin lymphoma. Blood 2009; 114: 2945–2951.
Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene 2006; 25: 2679–2684.
Kleppe M, Tousseyn T, Geissinger E, Kalender Atak Z, Aerts S, Rosenwald A et al. Mutation analysis of the tyrosine phosphatase PTPN2 in Hodgkin's lymphoma and T-cell non-Hodgkin's lymphoma. Haematologica 2011; 96: 1723–1727.
Tefferi A . Classification, diagnosis and management of myeloproliferative disorders in the JAK2V617F era. Hematology Am Soc Hematol Educ Program 2006. 240–245.
Kralovics R, Guan Y, Prchal JT . Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp Hematol 2002; 30: 229–236.
Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol 2011; 18: 971–976.
Argetsinger LS, Kouadio JL, Steen H, Stensballe A, Jensen ON, Carter-Su C . Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol Cell Biol 2004; 24: 4955–4967.
Feener EP, Rosario F, Dunn SL, Stancheva Z, Myers MG . Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol Cell Biol 2004; 24: 4968–4978.
Ishida-Takahashi R, Rosario F, Gong Y, Kopp K, Stancheva Z, Chen X et al. Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol Cell Biol 2006; 26: 4063–4073.
Lu X, Huang LJ, Lodish HF . Dimerization by a cytokine receptor is necessary for constitutive activation of JAK2V617F. J Biol Chem 2008; 283: 5258–5266.
Chen E, Beer PA, Godfrey AL, Ortmann CA, Li J, Costa-Pereira AP et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell 2010; 18: 524–535.
Staerk J, Kallin A, Demoulin JB, Vainchenker W . JAK1 Constantinescu SN and Tyk2 activation by the homologous polycythemia vera JAK2 V617F mutation: cross-talk with IGF1 receptor. J Biol Chem 2005; 280: 41893–41899.
Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL . JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood 2006; 108: 1652–1660.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 2008; 111: 3931–3940.
Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood 2008; 111: 3751–3759.
Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG . Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010; 115: 3589–3597.
Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 2010; 116: 1528–1538.
Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood 2010; 116: 783–787.
Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010; 17: 584–596.
Scott LM, Scott MA, Campbell PJ, Green AR . Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood 2006; 108: 2435–2437.
Dupont S, Masse A, James C, Teyssandier I, Lecluse Y, Larbret F et al. The JAK2 617V>F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood 2007; 110: 1013–1021.
Teofili L, Martini M, Cenci T, Petrucci G, Torti L, Storti S et al. Different STAT-3 and STAT-5 phosphorylation discriminates among Ph-negative chronic myeloproliferative diseases and is independent of the V617F JAK-2 mutation. Blood 2007; 110: 354–359.
Ugo V, Marzac C, Teyssandier I, Larbret F, Lecluse Y, Debili N et al. Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol 2004; 32: 179–187.
Garcon L, Rivat C, James C, Lacout C, Camara-Clayette V, Ugo V et al. Constitutive activation of STAT5 and Bcl-xL overexpression can induce endogenous erythroid colony formation in human primary cells. Blood 2006; 108: 1551–1554.
Walz C, Ahmed W, Lazarides K, Betancur M, Patel N, Hennighausen L et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2(V617F) in mice. Blood 2012; 119: 3550–3560.
Yan D, Hutchison RE, Mohi G . Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood 2012; 119: 3539–3549.
Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA 2006; 103: 1000–1005.
Hennighausen L, Robinson GW . Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev 2008; 22: 711–721.
Harrison C, Verstovsek S, McMullin MF, Mesa R . Janus kinase Inhibition and its effect upon the therapeutic landscape for myelofibrosis: from palliation to cure? Br J Haematol 2012; 157: 426–437.
Tefferi A . Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms. N Engl J Med 2012; 366: 844–846.
Tefferi AJAK . inhibitors for myeloproliferative neoplasms: clarifying facts from myths. Blood 2012; 119: 2721–2730.
Carbuccia N, Murati A, Trouplin V, Brecqueville M, Adelaide J, Rey J et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia 2009; 23: 2183–2186.
Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A et al. Mutation in TET2 in myeloid cancers. N Engl J Med 2009; 360: 2289–2301.
Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011; 478: 64–69.
Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med 2011; 365: 1384–1395.
Malcovati L, Della Porta MG, Pietra D, Boveri E, Pellagatti A, Galli A et al. Molecular and clinical features of refractory anemia with ringed sideroblasts associated with marked thrombocytosis. Blood 2009; 114: 3538–3545.
Hookham MB, Elliott J, Suessmuth Y, Staerk J, Ward AC, Vainchenker W et al. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood 2007; 109: 4924–4929.
Liu F, Zhao X, Perna F, Wang L, Koppikar P, Abdel-Wahab O et al. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell 2011; 19: 283–294.
Wrighton KH . Cell signalling: PRMT5 restricts ERK activity. Nat Rev Mol Cell Biol 2011; 12: 689.
Kanade SR, Eckert RL . Protein arginine methyltransferase 5 (PRMT5) signaling suppresses protein kinase Cdelta- and p38delta-dependent signaling and keratinocyte differentiation. J Biol Chem 2012; 287: 7313–7323.
Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 2009; 461: 819–822.
Besancenot R, Chaligne R, Tonetti C, Pasquier F, Marty C, Lecluse Y et al. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol 2010; 8: 9.
Pecquet C, Diaconu CC, Staerk J, Girardot M, Marty C, Royer Y et al. Thrombopoietin receptor down-modulation by JAK2 V617F: restoration of receptor levels by inhibitors of pathologic JAK2 signaling and of proteasomes. Blood 2012; 119: 4625–4635.
Marubayashi S, Koppikar P, Taldone T, Abdel-Wahab O, West N, Bhagwat N et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest 2010; 120: 3578–3593.
Dusa A, Mouton C, Pecquet C, Herman M, Constantinescu SN . JAK2 V617F constitutive activation requires JH2 residue F595: a pseudokinase domain target for specific inhibitors. PLoS One 2010; 5: e11157.
Dusa A, Staerk J, Elliott J, Pecquet C, Poirel HA, Johnston JA et al. Substitution of pseudokinase domain residue Val-617 by large non-polar amino acids causes activation of JAK2. J Biol Chem 2008; 283: 12941–12948.
Ma W, Kantarjian H, Zhang X, Yeh CH, Zhang ZJ, Verstovsek S et al. Mutation profile of JAK2 transcripts in patients with chronic myeloproliferative neoplasias. J Mol Diagn 2009; 11: 49–53.
Mead AJ, Rugless MJ, Jacobsen SE, Schuh A . Germline JAK2 mutation in a family with hereditary thrombocytosis. N Engl J Med 2012; 366: 967–969.
Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007; 356: 459–468.
Klampfl T, Harutyunyan A, Berg T, Gisslinger B, Schalling M, Bagienski K et al. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood 2011; 118: 167–176.
Passamonti F, Elena C, Schnittger S, Skoda RC, Green AR, Girodon F et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011; 117: 2813–2816.
Malinge S, Ben-Abdelali R, Settegrana C, Radford-Weiss I, Debre M, Beldjord K et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood 2007; 109: 2202–2204.
Kearney L, Gonzalez De Castro D, Yeung J, Procter J, Horsley SW, Eguchi-Ishimae M et al. Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood 2009; 113: 646–648.
Gaikwad A, Rye CL, Devidas M, Heerema NA, Carroll AJ, Izraeli S et al. Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br J Haematol 2009; 144: 930–932.
Mullighan CG . New strategies in acute lymphoblastic leukemia: translating advances in genomics into clinical practice. Clin Cancer Res 2011; 17: 396–400.
Chen IM, Harvey RC, Mullighan CG, Gastier-Foster J, Wharton W, Kang H et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children's Oncology Group study. Blood 2012; 119: 3512–3522.
Malinge S, Ragu C, Della-Valle V, Pisani D, Constantinescu SN, Perez C et al. Activating mutations in human acute megakaryoblastic leukemia. Blood 2008; 112: 4220–4226.
Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 2012; 481: 157–163.
Jeong EG, Kim MS, Nam HK, Min CK, Lee S, Chung YJ et al. Somatic mutations of JAK1 and JAK3 in acute leukemias and solid cancers. Clin Cancer Res 2008; 14: 3716–3721.
Hornakova T, Staerk J, Royer Y, Flex E, Tartaglia M, Constantinescu SN et al. Acute lymphoblastic leukemia-associated JAK1 mutants activate the Janus kinase/STAT pathway via interleukin-9 receptor alpha homodimers. J Biol Chem 2009; 284: 6773–6781.
Kleppe M, Soulier J, Asnafi V, Mentens N, Hornakova T, Knoops L et al. PTPN2 negatively regulates oncogenic JAK1 in T-cell acute lymphoblastic leukemia. Blood 2011; 117: 7090–7098.
Porcu M, Kleppe M, Gianfelici V, Geerdens E, De Keersmaecker K, Tartaglia M et al. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood 2012; 119: 4476–4479.
Kameda T, Shide K, Shimoda HK, Hidaka T, Kubuki Y, Katayose K et al. Absence of gain-of-function JAK1 and JAK3 mutations in adult T cell leukemia/lymphoma. Int J Hematol 2010; 92: 320–325.
Elliott NE, Cleveland SM, Grann V, Janik J, Waldmann TA, Dave UP . FERM domain mutations induce gain of function in JAK3 in adult T-cell leukemia/lymphoma. Blood 2011; 118: 3911–3921.
Riera L, Lasorsa E, Bonello L, Sismondi F, Tondat F, Di Bello C et al. Description of a novel Janus kinase 3 P132A mutation in acute megakaryoblastic leukemia and demonstration of previously reported Janus kinase 3 mutations in normal subjects. Leuk Lymphoma 2011; 52: 1742–1750.
Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell 2006; 10: 65–75.
Kaminker JS, Zhang Y, Waugh A, Haverty PM, Peters B, Sebisanovic D et al. Distinguishing cancer-associated missense mutations from common polymorphisms. Cancer Res 2007; 67: 465–473.
Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet 2009; 41: 446–449.
Olcaydu D, Harutyunyan A, Jager R, Berg T, Gisslinger B, Pabinger I et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet 2009; 41: 450–454.
Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet 2009; 41: 455–459.
Ritz O, Guiter C, Castellano F, Dorsch K, Melzner J, Jais JP et al. Recurrent mutations of the STAT6 DNA binding domain in primary mediastinal B-cell lymphoma. Blood 2009; 114: 1236–1242.
Baus D, Nonnenmacher F, Jankowski S, Doring C, Brautigam C, Frank M et al. STAT6 and STAT1 are essential antagonistic regulators of cell survival in classical Hodgkin lymphoma cell line. Leukemia 2009; 23: 1885–1893.
Nault JC, Fabre M, Couchy G, Pilati C, Jeannot E, Tran Van Nhieu J et al. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol 2012; 56: 184–191.
Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmaki H, Andersson EI et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. New Engl J Med 2012; 366: 1905–1913.
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006; 108: 3472–3476.
Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008; 112: 141–149.
Boyd EM, Bench AJ, Goday-Fernandez A, Anand S, Vaghela KJ, Beer P et al. Clinical utility of routine MPL exon 10 analysis in the diagnosis of essential thrombocythaemia and primary myelofibrosis. Br J Haematol 2010; 149: 250–257.
Pecquet C, Staerk J, Chaligne R, Goss V, Lee KA, Zhang X et al. Induction of myeloproliferative disorder and myelofibrosis by thrombopoietin receptor W515 mutants is mediated by cytosolic tyrosine 112 of the receptor. Blood 2010; 115: 1037–1048.
Staerk J, Lacout C, Sato T, Smith SO, Vainchenker W, Constantinescu SN . An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor. Blood 2006; 107: 1864–1871.
Ding J, Komatsu H, Wakita A, Kato-Uranishi M, Ito M, Satoh A et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood 2004; 103: 4198–4200.
Chaligne R, Tonetti C, Besancenot R, Roy L, Marty C, Mossuz P et al. New mutations of MPL in primitive myelofibrosis: only the MPL W515 mutations promote a G1/S-phase transition. Leukemia 2008; 22: 1557–1566.
Hussein K, Bock O, Theophile K, Schulz-Bischof K, Porwit A, Schlue J et al. MPLW515L mutation in acute megakaryoblastic leukaemia. Leukemia 2009; 23: 852–855.
Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, te Kronnie G et al. Gain-of-function mutations in interleukin-7 receptor-alpha (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med 2011; 208: 901–908.
Hertzberg L, Vendramini E, Ganmore I, Cazzaniga G, Schmitz M, Chalker J et al. Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood 2010; 115: 1006–1017.
Forbes LV, Gale RE, Pizzey A, Pouwels K, Nathwani A, Linch DC . An activating mutation in the transmembrane domain of the granulocyte colony-stimulating factor receptor in patients with acute myeloid leukemia. Oncogene 2002; 21: 5981–5989.
Plo I, Zhang Y, Le Couedic JP, Nakatake M, Boulet JM, Itaya M et al. An activating mutation in the CSF3R gene induces a hereditary chronic neutrophilia. J Exp Med 2009; 206: 1701–1707.
Beekman R, Touw IP . G-CSF and its receptor in myeloid malignancy. Blood 2010; 115: 5131–5136.
Beekman R, Valkhof MG, Sanders MA, van Strien PM, Haanstra JR, Broeders L et al. Sequential gain of mutations in severe congenital neutropenia progressing to acute myeloid leukemia. Blood 2012; 119: 5071–5077.
Golub TR, Barker GF, Lovett M, Gilliland DG . Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 1994; 77: 307–316.
Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 2003; 348: 1201–1214.
Sternberg DW, Gilliland DG . The role of signal transducer and activator of transcription factors in leukemogenesis. J Clin Oncol 2004; 22: 361–371.
Cain JA, Xiang Z, O'Neal J, Kreisel F, Colson A, Luo H et al. Myeloproliferative disease induced by TEL-PDGFRB displays dynamic range sensitivity to Stat5 gene dosage. Blood 2007; 109: 3906–3914.
Toffalini F, Demoulin JB . New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood 2010; 116: 2429–2437.
Choudhary C, Muller-Tidow C, Berdel WE, Serve H . Signal transduction of oncogenic Flt3. Int J Hematol 2005; 82: 93–99.
Choudhary C, Olsen JV, Brandts C, Cox J, Reddy PN, Bohmer FD et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell 2009; 36: 326–339.
Lasho T, Tefferi A, Pardanani A . Inhibition of JAK-STAT signaling by TG101348: a novel mechanism for inhibition of KITD816V-dependent growth in mast cell leukemia cells. Leukemia 2010; 24: 1378–1380.
Brender C, Lovato P, Sommer VH, Woetmann A, Mathiesen AM, Geisler C et al. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNalpha. Leukemia 2005; 19: 209–213.
Rudd CE . Lnk adaptor: novel negative regulator of B cell lymphopoiesis. Sci STKE 2001; 85: pe1.
Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci USA 2005; 102: 18962–18967.
Tong W, Lodish HF . Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J Exp Med 2004; 200: 569–580.
Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A et al. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med 2002; 195: 1599–1611.
Pardanani A, Lasho T, Finke C, Oh ST, Gotlib J, Tefferi A . LNK mutation studies in blast-phase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2 or MPL mutations. Leukemia 2010; 24: 1713–1718.
Bersenev A, Wu C, Balcerek J, Jing J, Kundu M, Blobel GA et al. Lnk constrains myeloproliferative diseases in mice. J Clin Invest 2010; 120: 2058–2069.
Krebs DL, Hilton DJ . SOCS: physiological suppressors of cytokine signaling. J Cell Sci 2000; 113 (Pt 16): 2813–2819.
Calabrese V, Mallette FA, Deschenes-Simard X, Ramanathan S, Gagnon J, Moores A et al. SOCS1 links cytokine signaling to p53 and senescence. Mol Cell 2009; 36: 754–767.
Melzner I, Bucur AJ, Bruderlein S, Dorsch K, Hasel C, Barth TF et al. Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in the MedB-1 mediastinal lymphoma line. Blood 2005; 105: 2535–2542.
Elliott J, Suessmuth Y, Scott LM, Nahlik K, McMullin MF, Constantinescu SN et al. SOCS3 tyrosine phosphorylation as a potential bio-marker for myeloproliferative neoplasms associated with mutant JAK2 kinases. Haematologica 2009; 94: 576–580.
Reddy J, Shivapurkar N, Takahashi T, Parikh G, Stastny V, Echebiri C et al. Differential methylation of genes that regulate cytokine signaling in lymphoid and hematopoietic tumors. Oncogene 2005; 24: 732–736.
Teofili L, Martini M, Cenci T, Guidi F, Torti L, Giona F et al. Epigenetic alteration of SOCS family members is a possible pathogenetic mechanism in JAK2 wild type myeloproliferative diseases. Int J Cancer 2008; 123: 1586–1592.
Jost E, do ON, Dahl E, Maintz CE, Jousten P, Habets L et al. Epigenetic alterations complement mutation of JAK2 tyrosine kinase in patients with BCR/ABL-negative myeloproliferative disorders. Leukemia 2007; 21: 505–510.
Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 2001; 409: 349–354.
Harashima A, Suzuki M, Okochi A, Yamamoto M, Matsuo Y, Motoda R et al. CD45 tyrosine phosphatase inhibits erythroid differentiation of umbilical cord blood CD34+ cells associated with selective inactivation of Lyn. Blood 2002; 100: 4440–4445.
Braggio E, Maiolino A, Gouveia ME, Magalhaes R, Souto Filho JT, Garnica M et al. Methylation status of nine tumor suppressor genes in multiple myeloma. Int J Hematol 2010; 91: 87–96.
Chim CS, Kwong YL, Liang R . Gene hypermethylation in multiple myeloma: lessons from a cancer pathway approach. Clin Lymphoma Myeloma 2008; 8: 331–339.
Liu X, Qu CK . Protein Tyrosine Phosphatase SHP-2 (PTPN11) in Hematopoiesis and Leukemogenesis. J Signal Transduct 2011: 195239.
Saur SJ, Sangkhae V, Geddis AE, Kaushansky K, Hitchcock IS . Ubiquitination and degradation of the thrombopoietin receptor c-Mpl. Blood 2010; 115: 1254–1263.
Bacher U, Haferlach C, Schnittger S, Kohlmann A, Kern W, Haferlach T . Mutations of the TET2 and CBL genes: novel molecular markers in myeloid malignancies. Ann Hematol 2010; 89: 643–652.
Kales SC, Ryan PE, Nau MM, Lipkowitz S . Cbl and human myeloid neoplasms: the Cbl oncogene comes of age. Cancer Res 2010; 70: 4789–4794.
Grand FH, Hidalgo-Curtis CE, Ernst T, Zoi K, Zoi C, McGuire C et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 2009; 113: 6182–6192.
Bommert K, Bargou RC, Stuhmer T . Signalling and survival pathways in multiple myeloma. Eur J Cancer 2006; 42: 1574–1580.
Ishikawa H, Tsuyama N, Abroun S, Liu S, Li FJ, Otsuyama K et al. Interleukin-6, CD45 and the src-kinases in myeloma cell proliferation. Leuk Lymphoma 2003; 44: 1477–1481.
Levy Y, Tsapis A, Brouet JC . Interleukin-6 antisense oligonucleotides inhibit the growth of human myeloma cell lines. J Clin Invest 1991; 88: 696–699.
Li J, Favata M, Kelley JA, Caulder E, Thomas B, Wen X et al. INCB16562, a JAK1/2 selective inhibitor, is efficacious against multiple myeloma cells and reverses the protective effects of cytokine and stromal cell support. Neoplasia 2010; 12: 28–38.
Ramakrishnan V, Kimlinger T, Haug J, Timm M, Wellik L, Halling T et al. TG101209, a novel JAK2 inhibitor, has significant in vitro activity in multiple myeloma and displays preferential cytotoxicity for CD45+ myeloma cells. Am J Hematol 2010; 85: 675–686.
Scuto A, Krejci P, Popplewell L, Wu J, Wang Y, Kujawski M et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia 2011; 25: 538–550.
Warsch W, Kollmann K, Eckelhart E, Fajmann S, Cerny-Reiterer S, Holbl A et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood 2011; 117: 3409–3420.
Hantschel O, Warsch W, Eckelhart E, Kaupe I, Grebien F, Wagner KU et al. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat Chem Biol 2012; 8: 285–293.
Ozawa Y, Williams AH, Estes ML, Matsushita N, Boschelli F, Jove R et al. Src family kinases promote AML cell survival through activation of signal transducers and activators of transcription (STAT). Leuk Res 2008; 32: 893–903.
Moucadel V, Constantinescu SN . Differential STAT5 signaling by ligand-dependent and constitutively active cytokine receptors. J Biol Chem 2005; 280: 13364–13373.
Girardot M, Pecquet C, Boukour S, Knoops L, Ferrant A, Vainchenker W et al. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood 2010; 116: 437–445.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 2011; 29: 789–796.
Santos FP, Kantarjian HM, Jain N, Manshouri T, Thomas DA, Garcia-Manero G et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood [Clinical Trial, Phase II Research Support, Non-U.S. Gov't] 2010; 115: 1131–1136.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. New Engl J Med 2010; 363: 1117–1127.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Verstovsek S . Janus-activated kinase 2 inhibitors: a new era of targeted therapies providing significant clinical benefit for Philadelphia chromosome-negative myeloproliferative neoplasms. J Clin Oncol 2011; 29: 781–783.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: 799–807.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: 787–798.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007; 1: 685–697.
Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 2007; 1: 671–684.
de Graaf CA, Kauppi M, Baldwin T, Hyland CD, Metcalf D, Willson TA et al. Regulation of hematopoietic stem cells by their mature progeny. Proc Natl Acad Sci USA 2010; 107: 21689–21694.
Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol 2010; 150: 446–455.
Hornakova T, Springuel L, Devreux J, Dusa A, Constantinescu SN, Knoops L et al. Oncogenic JAK1 and JAK2-activating mutations resistant to ATP-competitive inhibitors. Haematologica 2011; 96: 845–853.
Deshpande A, Reddy MM, Schade GO, Ray A, Chowdary TK, Griffin JD et al. Kinase domain mutations confer resistance to novel inhibitors targeting JAK2V617F in myeloproliferative neoplasms. Leukemia 2012; 26: 708–715.
Weigert O, Lane AA, Bird L, Kopp N, Chapuy B, van Bodegom D et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med 2012; 209: 259–273.
Guglielmelli P, Barosi G, Rambaldi A, Marchioli R, Masciulli A, Tozzi L et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood 2011; 118: 2069–2076.
Lu M, Wang J, Li Y, Berenzon D, Wang X, Mascarenhas J et al. Treatment with the Bcl-xL inhibitor ABT-737 in combination with interferon alpha specifically targets JAK2V617F-positive polycythemia vera hematopoietic progenitor cells. Blood 2010; 116: 4284–4287.
Guglielmelli P, Biamonte F, Score J, Hidalgo-Curtis C, Cervantes F, Maffioli M et al. EZH2 mutational status predicts poor survival in myelofibrosis. Blood 2011; 118: 5227–5234.
Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood 2011; 117: 3421–3429.
Acknowledgements
Financial support for medical editorial and graphic design assistance was provided by Novartis Pharmaceuticals. We thank Matthew Hoelzle PhD, Daniel Hutta, PhD and Isabelle Plo PhD for their assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
There is potential conflict of interest. Both WV and SNC have participated in ad-hoc Scientific Advisory Boards at Novartis related to JAK inhibitors.
Rights and permissions
About this article
Cite this article
Vainchenker, W., Constantinescu, S. JAK/STAT signaling in hematological malignancies. Oncogene 32, 2601–2613 (2013). https://doi.org/10.1038/onc.2012.347
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.347
Keywords
This article is cited by
-
Tyrosine phosphorylation of CARM1 promotes its enzymatic activity and alters its target specificity
Nature Communications (2024)
-
Killer cell lectin-like receptor G2 facilitates aggressive phenotypes of gastric cancer cells via dual activation of the ERK1/2 and JAK/STAT pathways
Gastric Cancer (2024)
-
A selective small-molecule STAT5 PROTAC degrader capable of achieving tumor regression in vivo
Nature Chemical Biology (2023)
-
Idasanutlin and navitoclax induce synergistic apoptotic cell death in T-cell acute lymphoblastic leukemia
Leukemia (2023)
-
miR-29b-3p suppresses the malignant biological behaviors of AML cells via inhibiting NF-κB and JAK/STAT signaling pathways by targeting HuR
BMC Cancer (2022)