Abstract
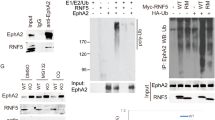
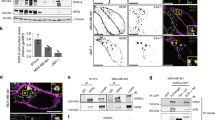
One of the causes of breast cancer is overexpression of the human epidermal growth factor receptor 2 (HER2). Enhanced receptor autophosphorylation and resistance to activation-induced downregulation have been suggested as mechanisms for HER2-induced sustained signaling and cell transformation. However, the molecular mechanisms underlying these possibilities remain incompletely understood. In the current report, we present evidence that show that HER2 overexpression does not lead to receptor hyper-autophosphorylation, but alters patterns in a manner that favors receptor stability and sustained signaling. Specifically, HER2 overexpression blocks epidermal growth factor receptor (EGFR) tyrosine phosphorylation on Y1045 and Y1068, the known docking sites of c-Cbl and Grb2, respectively, whereas promoting phosphorylation on Y1173, the known docking site of the Gab adaptor proteins and phospholipase C gamma. Under these conditions, HER2 itself is phosphorylated on Y1221/1222, with no known role, and on Y1248 that corresponds to Y1173 of EGFR. Interestingly, suppressed EGFR autophosphorylation on the Grb2 and c-Cbl-binding sites correlated with receptor stability and sustained signaling, suggesting that HER2 accomplishes these tasks by altering autophosphorylation patterns. In conformity with these findings, mutation of the Grb2-binding site on EGFR (Y1068F–EGFR) conferred resistance to ligand-induced degradation, which in turn induced sustained signaling, and increased cell proliferation and transformation. These findings suggest that the Grb2-binding site on EGFR is redundant for signaling, but critical for receptor regulation. On the other hand, mutation of the putative Grb2-binding site in HER2 (Y1139) did not affect stability, signaling or transformation, suggesting that Y1139 in HER2 may not serve as a Grb2-binding site. In agreement with the role of EGFR in HER2 signaling, inhibition of EGFR expression reduced HER2-induced anchorage-independent growth and tumorigenesis. These results imply that complementing HER2-targeted therapies with anti-EGFR drugs may be beneficial in HER2-positive breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dionne CA, Jaye M, Schlessinger J . Structural diversity and binding of FGF receptors. Ann N Y Acad Sci 1991; 638: 161–166.
Johnson DE, Williams LT . Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res 1993; 60: 1–41.
Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984; 309: 418–425.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 2003; 11: 495–505.
Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA . EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell 2003; 11: 507–517.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell 2002; 110: 763–773.
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002; 110: 775–787.
Badache A, Goncalves A . The ErbB2 signaling network as a target for breast cancer therapy. J Mammary Gland Biol Neoplasia 2006; 11: 13–25.
Carlsson J, Nordgren H, Sjostrom J, Wester K, Villman K, Bengtsson NO et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer 2004; 90: 2344–2348.
Fukushige S, Matsubara K, Yoshida M, Sasaki M, Suzuki T, Semba K et al. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol Cell Biol 1986; 6: 955–958.
King CR, Kraus MH, Aaronson SA . Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 1985; 229: 974–976.
Pawson T, Gish GD . SH2 and SH3 domains: from structure to function. Cell 1992; 71: 359–362.
Feng GS, Shen R, Heng HH, Tsui LC, Kazlauskas A, Pawson T . Receptor-binding tyrosine phosphorylation and chromosome localization of the mouse SH2-containing phosphotyrosine phosphatase Syp. Oncogene 1994; 9: 1545–1550.
Hadari YR, Kouhara H, Lax I, Schlessinger J . Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol 1998; 18: 3966–3973.
Lechleider RJ, Sugimoto S, Bennett AM, Kashishian AS, Cooper JA, Shoelson SE et al. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor. J Biol Chem 1993; 268: 21478–21481.
Schlessinger J, Mohammadi M, Margolis B, Ullrich A . Role of SH2-containing proteins in cellular signaling by receptor tyrosine kinases. Cold Spring Harb Symp Quant Biol 1992; 57: 67–74.
Agazie YM, Hayman MJ . Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol 2003; 23: 7875–7886.
Baldys A, Gooz M, Morinelli TA, Lee MH, Raymond JR, Luttrell LM et al. Essential role of c-Cbl in amphiregulin-induced recycling and signaling of the endogenous epidermal growth factor receptor. Biochemistry 2009; 48: 1462–1473.
Bollag G, McCormick F . Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature 1991; 351: 576–579.
Ekman S, Thuresson ER, Heldin CH, Ronnstrand L . Increased mitogenicity of an alphabeta heterodimeric PDGF receptor complex correlates with lack of RasGAP binding. Oncogene 1999; 18: 2481–2488.
Jiang X, Sorkin A . Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic 2003; 4: 529–543.
Pamonsinlapatham P, Hadj-Slimane R, Lepelletier Y, Allain B, Toccafondi M, Garbay C et al. P120-Ras GTPase activating protein (RasGAP): a multi-interacting protein in downstream signaling. Biochimie 2009; 91: 320–328.
Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 1997; 277: 333–338.
Beguinot L, Lyall RM, Willingham MC, Pastan I . Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci USA 1984; 81: 2384–2388.
Helin K, Beguinot L . Internalization and down-regulation of the human epidermal growth factor receptor are regulated by the carboxyl-terminal tyrosines. J Biol Chem 1991; 266: 8363–8368.
Sorkin A, Di Fiore PP, Carpenter G . The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene 1993; 8: 3021–3028.
Sorkin A, Goh LK . Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res 2009; 315: 683–696.
Huang G, Chantry A, Epstein RJ . Overexpression of ErbB2 impairs ligand-dependent downregulation of epidermal growth factor receptors via a post-transcriptional mechanism. J Cell Biochem 1999; 74: 23–30.
Worthylake R, Opresko LK, Wiley HS . ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem 1999; 274: 8865–8874.
Zhou X, Agazie YM . The signaling and transformation potency of the overexpressed HER2 protein is dependent on the normally-expressed EGFR. Cell Signal 2012; 24: 140–150.
Hendriks BS, Wiley HS, Lauffenburger D . HER2-mediated effects on EGFR endosomal sorting: analysis of biophysical mechanisms. Biophys J 2003; 85: 2732–2745.
Muthuswamy SK, Gilman M, Brugge JS . Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol 1999; 19: 6845–6857.
Jiang X, Huang F, Marusyk A, Sorkin A . Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol Biol Cell 2003; 14: 858–870.
Sun J, Pedersen M, Bengtsson S, Ronnstrand L . Grb2 mediates negative regulation of stem cell factor receptor/c-Kit signaling by recruitment of Cbl. Exp Cell Res 2007; 313: 3935–3942.
Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH . Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol 2002; 156: 843–854.
Aertgeerts K, Skene R, Yano J, Sang BC, Zou H, Snell G et al. Structural analysis of the mechanism of inhibition and allosteric activation of the kinase domain of HER2 protein. J Biol Chem 2011; 286: 18756–18765.
Fan YX, Wong L, Ding J, Spiridonov NA, Johnson RC, Johnson GR . Mutational activation of ErbB2 reveals a new protein kinase autoinhibition mechanism. J Biol Chem 2008; 283: 1588–1596.
Morandell S, Stasyk T, Skvortsov S, Ascher S, Huber LA . Quantitative proteomics and phosphoproteomics reveal novel insights into complexity and dynamics of the EGFR signaling network. Proteomics 2008; 8: 4383–4401.
Ono M, Kuwano M . Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin Cancer Res 2006; 12: 7242–7251.
Fragale A, Tartaglia M, Wu J, Gelb BD . Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum Mutat 2004; 23: 267–277.
Meng S, Chen Z, Munoz-Antonia T, Wu J . Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochem J 2005; 391: 143–151.
Ravichandran KS, Lorenz U, Shoelson SE, Burakoff SJ . Interaction of Shc with Grb2 regulates the Grb2 association with mSOS. Ann N Y Acad Sci 1995; 766: 202–203.
Russo C, Dolcini V, Salis S, Venezia V, Violani E, Carlo P et al. Signal transduction through tyrosine-phosphorylated carboxy-terminal fragments of APP via an enhanced interaction with Shc/Grb2 adaptor proteins in reactive astrocytes of Alzheimer's disease brain. Ann N Y Acad Sci 2002; 973: 323–333.
Schulze WX, Deng L, Mann M . Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol 2005; 1: 0001–0008.
Monsey J, Shen W, Schlesinger P, R Bose . Her4 and Her2/neu tyrosine kinase domains dimerize and activate in a reconstituted in vitro system. J Biol Chem 2010; 285: 7035–7044.
Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J . An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006; 125: 1137–1149.
Yamanashi Y, Tezuka T, Yokoyama K . Activation of receptor protein-tyrosine kinases from the cytoplasmic compartment. J Biochem. 151: 353–359.
Zhou X, Agazie YM . Molecular mechanism for SHP2 in promoting HER2-induced signaling and transformation. J Biol Chem 2009; 284: 12226–12234.
Sperka T, Geissler KJ, Merkel U, Scholl I, Rubio I, Herrlich P et al. Activation of Ras requires the ERM-dependent link of actin to the plasma membrane. PLoS One 2011; 6: e27511.
Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 2008; 68: 5878–5887.
Rana P, Sridhar SS . Efficacy and tolerability of lapatinib in the management of breast cancer. Breast Cancer (Auckl) 6: 67–77.
Debnath J, Muthuswamy SK, Brugge JS . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30: 256–268.
Agazie YM, Movilla N, Ischenko I, Hayman MJ . The phosphotyrosine phosphatase SHP2 is a critical mediator of transformation induced by the oncogenic fibroblast growth factor receptor 3. Oncogene 2003; 22: 6909–6918.
Yuan YL, Zhou XH, Song J, Qiu XP, Li J, Ye LF . Dual silencing of type 1 insulin-like growth factor and epidermal growth factor receptors to induce apoptosis of nasopharyngeal cancer cells. J Laryngol Otol 2008; 122: 952–960.
Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL et al. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem 2003; 278: 28950–28960.
Acknowledgements
This work was supported by a grant number CA124940 from the National Cancer Institute (NCI), a component of the National Institute of Health (NIH) to YMA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hartman, Z., Zhao, H. & Agazie, Y. HER2 stabilizes EGFR and itself by altering autophosphorylation patterns in a manner that overcomes regulatory mechanisms and promotes proliferative and transformation signaling. Oncogene 32, 4169–4180 (2013). https://doi.org/10.1038/onc.2012.418
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.418
Keywords
This article is cited by
-
Conditional knockout of SHP2 in ErbB2 transgenic mice or inhibition in HER2-amplified breast cancer cell lines blocks oncogene expression and tumorigenesis
Oncogene (2019)
-
Assessing metastatic potential of breast cancer cells based on EGFR dynamics
Scientific Reports (2019)
-
Molecular dynamics simulations of asymmetric heterodimers of HER1/HER2 complexes
Journal of Molecular Modeling (2018)
-
The ubiquitin-proteasome pathway in adult and pediatric brain tumors: biological insights and therapeutic opportunities
Cancer and Metastasis Reviews (2017)
-
SHP2 acts both upstream and downstream of multiple receptor tyrosine kinases to promote basal-like and triple-negative breast cancer
Breast Cancer Research (2016)