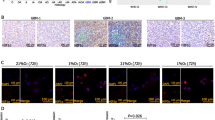
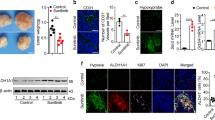
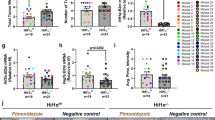
Abstract
Hypoxia is a salient feature of most solid tumors, and hypoxic adaptation of cancer cells has crucial implications in propagation of malignant clonal cell population. Osteopontin (OPN) has been identified as a hypoxia-responsive gene, but the mechanistic and regulatory role of OPN under hypoxia is less characterized. The present study identifies the existence of a positive inter-regulatory loop between hypoxia and OPN. We have shown that hypoxia induces OPN expression in breast cancer cells; however, the expression was found to be HIF1α independent. OPN enabled transcriptional upregulation of HIF1α expression both under normoxia and hypoxia, whereas stability of HIF1α protein in breast cancer cells remained unaffected. Moreover, we have shown that OPN induces integrin-linked kinase (ILK)/Akt-mediated nuclear factor (NF)-κB p65 activation leading to HIF1α-dependent vascular endothelial growth factor (VEGF) expression and angiogenesis in response to hypoxia. These in vitro data are biologically important as OPN expressing cells induce greater tumor growth and angiogenesis through enhanced expressions of proangiogenic molecules as compared with control. Immunohistochemical analysis of human breast cancer specimens revealed significant correlation between OPN and HIF1α but not HIF2α. Elevated expression of HIF1α and OPN was observed in pre-neoplastic and early stage infiltrating ductal carcinoma implicating the role of these proteins in neoplastic progression of breast cancer. Together, our results substantiate the prime role of OPN in cellular adaptation through ILK and NF-κB-mediated HIF1α-dependent VEGF expression in response to hypoxia that ultimately controls breast cancer progression and angiogenesis. Our study reinforces the fact that targeting OPN and its regulated signaling network hold important therapeutic implications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Harris AL . Hypoxia—a key regulatory factor in tumor growth. Nat Rev Cancer 2002; 2: 38–47.
Semenza GL . Hypoxia, clonal selection, and the role of HIF1 in tumor progression. Crit Rev Biochem Mol Biol 2000; 35: 71–103.
Vaupel P . Metabolic microenvironment of tumor cells: a key factor in malignant progression. Exp Oncol 2010; 32: 125–127.
Subarsky P, Hill RP . The hypoxic tumor microenvironment and metastatic progression. Clin Exp Metastasis 2003; 20: 237–250.
Vaupel P, Harrison L . Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 2004; 9: 4–9.
Loboda A, Jozkowicz A, Dulak J . HIF-1 and HIF-2 transcription factors-similar but not identical. Mol Cells 2010; 29: 435–442.
Hammond EM, Giaccia AJ . Hypoxia-inducible factor-1 and p53: friends, acquaintances or strangers? Clin Cancer Res 2006; 12: 5007–5009.
Chan DA, Giaccia AJ . PHD2 in tumor angiogenesis. Br J Cancer 2010; 103: 1–5.
Keith B, Johnson RS, Simon MC . HIF1α and HIF2α: sibling rivalry in hypoxic tumor growth and progression. Nat Rev Cancer 2011; 12: 9–22.
Hockel M, Vaupel P . Biological consequence of tumor hypoxia. Semin Oncol 2001; 28: 36–41.
Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene 2003; 22: 5907–5914.
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zaqzaq D et al. Overexpression of hypoxia-inducible factor1α in common human cancers and their metastases. Cancer Res 1999; 59: 5830–5835.
Bos R, Van der Groep P, Greijer AE, Shvartz A, Meijer S, Pinedo HM et al. Levels of hypoxia-inducible factor 1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 2003; 97: 1573–1581.
Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P . Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56: 4509–4515.
Vaupel P, Mayer A, Briest S, Hockel M . Oxygenation gain factor: a novel parameter characterizing the association between hemoglobin level and the oxygenation status of breast cancers. Cancer Res 2003; 63: 7634–7637.
Kaidi A, Qualtrough D, Williams AC, Paraskeva C . Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor 1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res 2006; 66: 6683–6691.
Doe MR, Ascano JM, Kaur M, Cole MD . Myc postranscriptionally induces HIF1 protein and target gene expression in normal and cancer cells. Cancer Res 2012; 72: 949–957.
Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL . HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α synthesis: novel mechanism for HIF-1 mediated vascular endothelial growth factor expression. Mol Cell Biol 2001; 21: 3995–4004.
Rangaswami H, Bulbule A, Kundu GC . Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol 2006; 16: 79–87.
Rittling SR, Chambers AF . Role of osteopontin in tumor progression. Br J Cancer 2004; 90: 1877–1881.
Chakraborty G, Jain S, Behera R, Ahmed M, Sharma P, Kumar V et al. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr Mol Med 2006; 6: 819–830.
Wai PY, Kuo PC . The role of Osteopontin in tumor metastasis. J Surg Res 2004; 121: 228–241.
Cook AC, Tuck AB, McCarthy S, Turner JG, Irby RB, Bloom GC et al. OPN induces multiple changes in gene expression that reflect the six “hallmarks of cancer” in a model of breast cancer progression. Mol Carcinog 2005; 43: 225–236.
Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW . Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate and lung cancer. Clin Cancer Res 2001; 7: 4060–4066.
Jain A, McKnight DA, Fisher LW, Humphreys EB, Mangold LA, Partin AW et al. Small integrin-binding proteins as serum markers for prostate cancer detection. Clin Cancer Res 2009; 15: 5199–5207.
Singhal H, Bautista DS, Tonkin KS, O’Malley FP, Tuck AB, Chambers AF et al. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res 1997; 3: 605–611.
Ahmed M, Behera R, Chakraborty G, Jain S, Kumar V, Sharma P et al. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Targets 2011; 15: 1113–1126.
McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 2008; 133: 994–1005.
Zhu Y, Denhardt DT, Cao H, Sutphin PD, Koong AC, Giaccia AJ et al. Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer. Oncogene 2005; 24: 6555–6563.
Le QT, Sutphin PD, Raychaudhuri S, Yu SC, Terris DJ, Lin HS et al. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res 2003; 9: 59–67.
Kim HJ, Lee HJ, Jun JI, Oh Y, Choi SG, Kim H et al. Intracellular cleavage of osteopontin by caspase 8 modulates hypoxia/reoxygenation cell death through p53. Proc Natl Acad Sci USA 2009; 106: 15326–15331.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS . Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 2001; 107: 1055–1061.
Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC . Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol Biol Cell 2005; 16: 1901–1912.
Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C et al. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res 2009; 69: 3308–3316.
Janji B, Melchior C, Vallar L, Kieffer N . Cloning of an isoform of integrin-linked kinase (ILK) that is upregulated in HT-144 melanoma cells following TGF-β1 stimulation. Oncogene 2000; 19: 3069–3077.
Song G, Ouyang G, Mao Y, Ming Y, Bao S, Hu T . Osteopontin promotes gastric cancer metastasis by augmenting cell survival and invasion through Akt-mediated HIF-1alpha up-regulation and MMP9 activation. J Cell Mol Med 2009; 13: 1706–1718.
Yang L, Zhao WS, Zuo WS, Wei L, Song XR, Wang XW et al. Silencing of osteopontin promotes the radiosensitivity of breast cancer cells by reducing the expression of hypoxia-inducible factor 1 and vascular endothelial growth factor. Chin Med J 2012; 125: 293–299.
Stasinopoulos I, O’Brien DR, Bhujwalla ZM . Inflammation but not hypoxia, mediated HIF1α activation depends on COX-2. Cancer Biol Ther 2009; 8: 31–35.
Said HM, Hagemann C, Stojic J, Schoemig B, Vince GH, Flentje M et al. GAPDH is not regulated in human glioblastoma under hypoxic conditions. BMC Mol Biol 2007; 8: 55.
Kuo HP, Lee DF, Xia W, Wei Y, Hung MC . TNF-α induces HIF1α expression through activation of IKKβ. Biochem Biophys Res Commun 2009; 389: 640–644.
Brahimi Horn C, Mazure N, Pouyssegur J . Signalling via the hypoxia-inducible factor 1α requires multiple post translational modifications. Cell Signal 2005; 17: 1–9.
Chen R, Liliental JE, Kowalski PE, Lu Q, Cohen SN . Regulation of transcription of hypoxia-inducible factor 1 α (HIF1 α) by heat shock factors HSF2 and HSF4. Oncogene 2011; 30: 2570–2580.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M et al. HIF α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292: 464–468.
Vivanco I, Sawyers CL . The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501.
Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A . Akt1 activation can augment hypoxia-inducible factor-1α expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Mol Cancer Res 2006; 7: 471–479.
Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA 2000; 97: 3207–3212.
Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K et al. Regulation of tumor angiogenesis by integrin-linked kinase (ILK). Cancer Cell 2004; 5: 79–90.
Bendinelli P, Matteucci E, Maroni P, Desiderio MA . NF-κB activation, dependent on acetylation/deacetylation, contributes to HIF-1 activity and migration of bone metastatic breast carcinoma cells. Mol Cancer Res 2009; 7: 1328–1341.
Van Uden P, Kenneth NS, Rocha S . Regulation of hypoxia inducible factor -1α by NF-κB. Biochem J 2008; 412: 477–484.
Liao D, Corle C, Seagroves TN, Johnson RS . Hypoxia-inducible factor-1α is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res 2007; 67: 563–572.
Mukundan H, Kanagy NL, Resta TC . 17-beta estradiol attenuates hypoxic induction of HIF-1alpha and erythropoietin in Hep3B cells. J Cardiovasc Pharmacol 2004; 44: 93–100.
Machado-Linde F, Pelegrin P, Sanchez-Ferrer ML, Leon J, Cascales P, Parrilla JJ . 2-Methoxyestradiol in the pathophysiology of endometriosis: focus on angiogenesis and therapeutic potential. Reprod Sci 2012; 19: 1018–1029.
Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P et al. HIF1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA 2011; 108: 16369–16374.
Gu T, Ohashi R, Cui R, Tajima K, Yoshioka M, Iwakami S et al. Osteopontin is involved in the development of acquired chemo-resistance of cisplatin in small cell lung cancer. Lung Cancer 2009; 66: 176–183.
Rohwer N, Dame C, Haugstetter A, Weidenmann B, Detjen K, Schmitt CA et al. Hypoxia-inducible factor 1α determines gastric cancer chemosensitivity via modulation of p53 and NF-κB. Plos One 2010; 5: e12038.
Miller RL, Kohan DE . Hypoxia regulates endothelin-1 production by the inner medullary collecting duct. J Lab Clin Med 1998; 131: 45–48.
Lech M, Avila-Ferrufino A, Allam R, Segerer S, Khandoga A, Krombach F et al. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol 2009; 183: 4109–4118.
Mihai LG, Mitrea N, Papacocea RI, Badarau AI . Dynamic activity of some antioxidant enzymes in primary cultures of neurons under hypoxia and ischemia. Farmacia 2012; 60: 49–57.
Lu ZH, Books JT, Ley TJ . YB-1 is important for late stage embryonic development, optimal cellular stress responses, and the prevention of premature senescence. Mol Cell Biol 2005; 25: 4625–4637.
Ahmed M, Kundu GC . Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NFκB dependent AP-1 mediated ICAM-1 expression in breast cancer cells. Mol Cancer 2010; 9: 101–113.
Kumar V, Behera R, Lohite K, Karnik S, Kundu GC . p38 kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer Res 2010; 70: 10381–10391.
Behera R, Kumar V, Lohite K, Karnik S, Kundu GC . Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis 2010; 31: 192–200.
Sliwa M, Markovic D, Gabrusiewicz K, Sinowitz M, Glass R, Zawadzka M et al. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain 2007; 130: 476–489.
Chakraborty G, Jain S, Kundu GC . Osteopontin promotes vascular endothelial growth factor–dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res 2008; 68: 152–161.
Jain S, Chakraborty G, Raja R, Kale S, Kundu GC . Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res 2008; 68: 7750–7759.
Sharma P, Kumar S, Kundu GC . Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells. Mol Cancer 2010; 9: 178.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Acknowledgements
We thank Dr B. Ramanamurthy, In-charge, Experimental Animal Facility (EAF), NCCS, India for breeding and maintaining NOD-SCID and OPN KO mice. We also thank Dr S. Ghaskabdi, Department of Zoology, University of Pune, India for providing the facility for CAM assay. This work was supported by grants from Council of Scientific and Industrial Research (CSIR) (to GCK, SK and GS) and Indian Council of Medical Research (ICMR) (to RR). This project was funded by CSIR, ICMR and NCCS, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Raja, R., Kale, S., Thorat, D. et al. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1α-mediated VEGF-dependent angiogenesis. Oncogene 33, 2053–2064 (2014). https://doi.org/10.1038/onc.2013.171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.171
Keywords
This article is cited by
-
Osteopontin secreted from obese adipocytes enhances angiogenesis and promotes progression of pancreatic ductal adenocarcinoma in obesity
Cellular Oncology (2024)
-
Osteopontin: A Novel Therapeutic Target for Respiratory Diseases
Lung (2024)
-
Targeting hypoxia in solid and haematological malignancies
Journal of Experimental & Clinical Cancer Research (2022)
-
Hepatic stellate cells promote intrahepatic cholangiocarcinoma progression via NR4A2/osteopontin/Wnt signaling axis
Oncogene (2021)
-
Understanding breast cancer heterogeneity through non-genetic heterogeneity
Breast Cancer (2021)