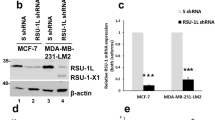
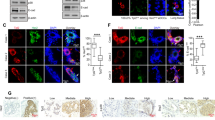
Abstract
Metastasis suppressors comprise a growing class of genes whose downregulation triggers metastatic progression. In contrast to tumor suppressors, metastasis suppressors are rarely mutated or deleted, and little is known regarding the mechanisms by which their expression is downregulated. Here, we demonstrate that the metastasis suppressor, NM23-H1, is degraded by lysosomal cysteine cathepsins (L,B), which directly cleave NM23-H1. In addition, activation of c-Abl and Arg oncoproteins induces NM23-H1 degradation in invasive cancer cells by increasing cysteine cathepsin transcription and activation. Moreover, c-Abl activates cathepsins by promoting endosome maturation, which facilitates trafficking of NM23-H1 to the lysosome where it is degraded. Importantly, the invasion- and metastasis-promoting activity of c-Abl/Arg is dependent on their ability to induce NM23-H1 degradation, and the pathway is clinically relevant as c-Abl/Arg activity and NM23-H1 expression are inversely correlated in primary breast cancers and melanomas. Thus, we demonstrate a novel mechanism by which cathepsin expression is upregulated in cancer cells (via Abl kinases). We also identify a novel role for intracellular cathepsins in invasion and metastasis (degradation of a metastasis suppressor). Finally, we identify novel crosstalk between oncogenic and metastasis suppressor pathways, thereby providing mechanistic insight into the process of NM23-H1 loss, which may pave the way for new strategies to restore NM23-H1 expression and block metastatic progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Novak M, Jarrett SG, McCorkle JR, Mellon I, Kaetzel DM . Multiple mechanisms underlie metastasis suppressor function of NM23-H1 in melanoma. Naunyn Schmiedebergs Arch Pharmacol 2011; 384: 433–438.
Steeg PS, Horak CE, Miller KD . Clinical-translational approaches to the Nm23-H1 metastasis suppressor. Clin Cancer Res 2008; 14: 5006–5012.
Smith SC, Theodorescu D . Learning therapeutic lessons from metastasis suppressor proteins. Nat Rev Cancer 2009; 9: 253–264.
Saha A, Robertson ES . Functional modulation of the metastatic suppressor Nm23-H1 by oncogenic viruses. FEBS Lett 2011; 585: 3174–3184.
Horak CE, Lee JH, Marshall JC, Shreeve SM, Steeg PS . The role of metastasis suppressor genes in metastatic dormancy. APMIS 2008; 116: 586–601.
Boissan M, De Wever O, Lizarraga F, Wendum D, Poincloux R, Chignard N et al. Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res 2010; 70: 7710–7722.
Marshall JC, Collins J, Marino N, Steeg P . The Nm23-H1 metastasis suppressor as a translational target. Eur J Cancer 2010; 46: 1278–1282.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta 2012; 1824: 68–88.
Reiser J, Adair B, Reinheckel T . Specialized roles for cysteine cathepsins in health and disease. J Clin Invest 2010; 120: 3421–3431.
De Braekeleer E, Douet-Guilbert N, Rowe D, Bown N, Morel F, Berthou C et al. ABL1 fusion genes in hematological malignancies: a review. Eur J Haematol 2011; 86: 361–371.
Srinivasan D, Plattner R . Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res 2006; 66: 5648–5655.
Srinivasan D, Sims JT, Plattner R . Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene 2008; 27: 1095–1105.
Srinivasan D, Kaetzel DM, Plattner R . Reciprocal regulation of Abl and receptor tyrosine kinases. Cell Signal 2009; 21: 1143–1150.
Ganguly SS, Fiore LS, Sims JT, Friend JW, Srinivasan D, Thacker MA et al. c-Abl and Arg are activated in human primary melanomas, promote melanoma cell invasion via distinct pathways, and drive metastatic progression. Oncogene 2012; 31: 1804–1816.
Ganguly SS, Plattner R . Activation of Abl family kinases in solid tumors. Genes Cancer 2012; 3: 414–425.
Iizuka N, Oka M, Noma T, Nakazawa A, Hirose K, NM23-H1 Suzuki T . NM23-H1 and NM23-H2 messenger RNA abundance in human hepatocellular carcinoma. Cancer Res 1995; 55: 652–657.
Ma D, Luyten GP, Luider TM, Jager MJ, Niederkorn JY . Association between NM23-H1 gene expression and metastasis of human uveal melanoma in an animal model. Invest Ophthalmol Vis Sci 1996; 37: 2293–2301.
Easty DJ, Maung K, Lascu I, Veron M, Fallowfield ME, Hart IR et al. Expression of NM23 in human melanoma progression and metastasis. Br J Cancer 1996; 74: 109–114.
Goodall RJ, Dawkins HJ, Robbins PD, Hahnel E, Sarna M, Hahnel R et al. Evaluation of the expression levels of nm23-H1 mRNA in primary breast cancer, benign breast disease, axillary lymph nodes and normal breast tissue. Pathology 1994; 26: 423–428.
Hwang BG, Park IC, Park MJ, Moon NM, Choi DW, Hong WS et al. Role of the nm23-H1 gene in the metastasis of gastric cancer. J Korean Med Sci 1997; 12: 514–518.
Myeroff LL, Markowitz SD . Increased nm23-H1 and nm23-H2 messenger RNA expression and absence of mutations in colon carcinomas of low and high metastatic potential. J Natl Cancer Inst 1993; 85: 147–152.
Sgouros J, Galani E, Gonos E, Moutsatsou P, Belechri M, Skarlos D et al. Correlation of nm23-H1 gene expression with clinical outcome in patients with advanced breast cancer. In Vivo 2007; 21: 519–522.
Lin LI, Lee PH, Wu CM, Lin JK . Significance of nm23 mRNA expression in human hepatocellular carcinoma. Anticancer Res 1998; 18: 541–546.
Engel M, Theisinger B, Seib T, Seitz G, Huwer H, Zang KD et al. High levels of nm23-H1 and nm23-H2 messenger RNA in human squamous-cell lung carcinoma are associated with poor differentiation and advanced tumor stages. Int J Cancer 1993; 55: 375–379.
Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD . MDA-MB-435 cells are derived from M14 melanoma cells—a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat 2007; 104: 13–19.
Chambers AF . MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res 2009; 69: 5292–5293.
Jacob F, Guertler R, Naim S, Nixdorf S, Fedier A, Hacker NF et al. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS One 2013; 8: e59180.
Yogalingam G, Pendergast AM . Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components. J Biol Chem 2008; 283: 35941–35953.
Barila D, Superti-Furga G . An intramolecular SH3-domain interaction regulates c-Abl activity. Nature Genet 1998; 18: 280–282.
Huotari J, Helenius A . Endosome maturation. EMBO J 2011; 30: 3481–3500.
Mesaki K, Tanabe K, Obayashi M, Oe N, Takei K . Fission of tubular endosomes triggers endosomal acidification and movement. PLoS One 2011; 6: e19764.
Press B, Feng Y, Hoflack B, Wandinger-Ness A . Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol 1998; 140: 1075–1089.
De Wever O, Hendrix A, De Boeck A, Westbroek W, Braems G, Emami S et al. Modeling and quantification of cancer cell invasion through collagen type I matrices. Int J Dev Biol 2010; 54: 887–896.
McDermott WG, Boissan M, Lacombe ML, Steeg PS, Horak CE . Nm23-H1 homologs suppress tumor cell motility and anchorage independent growth. Clin Exp Metastasis 2008; 25: 131–138.
Horak CE, Lee JH, Elkahloun AG, Boissan M, Dumont S, Maga TK et al. Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res 2007; 67: 7238–7246.
Allington TM, Galliher-Beckley AJ, Schiemann WP . Activated Abl kinase inhibits oncogenic transforming growth factor-beta signaling and tumorigenesis in mammary tumors. FASEB J 2009; 23: 4231–4243.
Allington TM, Schiemann WP . The Cain and Abl of epithelial-mesenchymal transition and transforming growth factor-beta in mammary epithelial cells. Cells Tissues Organs 2011; 193: 98–113.
Wright PK . Targeting vesicle trafficking: an important approach to cancer chemotherapy. Recent Pat Anticancer Drug Discov 2008; 3: 137–147.
Rosenfeld JL, Moore RH, Zimmer KP, Alpizar-Foster E, Dai W, Zarka MN et al. Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J Cell Sci 2001; 114 (Pt 24): 4499–4508.
Rotty JD, Wu C, Bear JE . New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol 2013; 14: 7–12.
Sossey-Alaoui K, Li X, Cowell JK . c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J Biol Chem 2007; 282: 26257–26265.
Curtis CD, Likhite VS, McLeod IX, Yates JR, Nardulli AM . Interaction of the tumor metastasis suppressor nonmetastatic protein 23 homologue H1 and estrogen receptor alpha alters estrogen-responsive gene expression. Cancer Res 2007; 67: 10600–10607.
Platta HW, Stenmark H . Endocytosis and signaling. Curr Opin Cell Biol 2011; 23: 393–403.
Woolworth JA, Nallamothu G, Hsu T . The Drosophila metastasis suppressor gene Nm23 homolog, awd, regulates epithelial integrity during oogenesis. Mol Cell Biol 2009; 29: 4679–4690.
Krishnan KS, Rikhy R, Rao S, Shivalkar M, Mosko M, Narayanan R et al. Nucleoside diphosphate kinase, a source of GTP, is required for dynamin-dependent synaptic vesicle recycling. Neuron 2001; 30: 197–210.
Annesley SJ, Bago R, Bosnar MH, Filic V, Marinovic M, Weber I et al. Dictyostelium discoideum nucleoside diphosphate kinase C plays a negative regulatory role in phagocytosis, macropinocytosis and exocytosis. PLoS One 2011; 6: e26024α.
Palacios F, Schweitzer JK, Boshans RL, D'Souza-Schorey C . ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 2002; 4: 929–936.
Hsu T, Adereth Y, Kose N, Dammai V . Endocytic function of von Hippel-Lindau tumor suppressor protein regulates surface localization of fibroblast growth factor receptor 1 and cell motility. J Biol Chem 2006; 281: 12069–12080.
Jacob M, Todd LA, Majumdar RS, Li Y, Yamamoto K, Pure E . Endogenous cAbl regulates receptor endocytosis. Cell Signal 2009; 21: 1308–1316.
Wetzel DM, McMahon-Pratt D, Koleske AJ . The Abl and Arg kinases mediate distinct modes of phagocytosis and are required for maximal Leishmania infection. Mol Cell Biol 2012; 32: 3176–3186.
Xiong W, Morillo SA, Rebay I . The Abelson tyrosine kinase regulates Notch endocytosis and signaling to maintain neuronal cell fate in Drosophila photoreceptors. Development 2012; 140: 176–186.
Tanos B, Pendergast AM . Abl tyrosine kinase regulates endocytosis of the epidermal growth factor receptor. J Biol Chem 2006; 281: 32714–32723.
Bauer B, Bartfeld S, Meyer TF . H. pylori selectively blocks EGFR endocytosis via the non-receptor kinase c-Abl and CagA. Cell Microbiol 2009; 11: 156–169.
Balaji K, Mooser C, Janson CM, Bliss JM, Hojjat H, Colicelli J . RIN1 orchestrates the activation of RAB5 GTPases and ABL tyrosine kinases to determine EGFR fate. J Cell Sci 2012; 125 (Pt 23): 5887–5896.
Huang S, DeGuzman A, Bucana CD, Fidler IJ . Level of interleukin-8 expression by metastatic human melanoma cells directly correlates with constitutive NF-kappaB activity. Cytokines Cell Mol Ther 2000; 6: 9–17.
Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, Chanock SJ et al. DNA fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther 2009; 8: 713–724.
Sims JT, Ganguly SS, Bennett H, Friend WJ, J. T, Plattner R . Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-κB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS One 2013; 8: e55509.
Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest 2005; 115: 3166–3176.
Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM . c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev 1999; 13: 2400–2411.
Ma D, McCorkle JR, Kaetzel DM . The metastasis suppressor NM23-H1 possesses 3'-5' exonuclease activity. J Biol Chem 2004; 279: 18073–18084.
Taha TA, El-Alwani M, Hannun YA, Obeid LM . Sphingosine kinase-1 is cleaved by cathepsin B in vitro: identification of the initial cleavage sites for the protease. FEBS Lett 2006; 580: 6047–6054.
Authier F, Metioui M, Bell AW, Mort JS . Negative regulation of epidermal growth factor signaling by selective proteolytic mechanisms in the endosome mediated by cathepsin B. J Biol Chem 1999; 274: 33723–33731.
Fenyo D, Wang Q, DeGrasse JA, Padovan JC, Cadene M, Chait BT . MALDI sample preparation: the ultra thin layer method. J Vis Exp 2007; 3: 192.
Mitra S, Beach C, Feng GS, Plattner R . SHP-2 is a novel target of Abl kinases during cell proliferation. J Cell Sci 2008; 121 (Pt 20): 3335–3346.
Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A . Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 2004; 313: 856–862.
Acknowledgements
We thank the following individuals for their assistance. Garretson Epperly and James Begley (confocal), Spear/Peterson Labs (qPCR), Jonathan Sims and Divya Srinivasan (cell lysates), Vivek Rangnekar, Suzanne Ridges, Aditi Jain (manuscript review) and Woodrow Friend (Figures 1a and 6d). Mass spectrometry was performed at the University of Kentucky Proteomics Core Facility, which is supported in part by the Office of the Vice President for Research. Edman Degradation was performed at the University of Nebraska Protein Structure Core Facility. This work was supported by NIH/NCI/R01 grants to RP (CA116784, CA166499). Dr Plattner is funded by NIH/NCI. DMK also is funded by NIH/NCI, but this work was not supported by his grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
LSF performed experiments for Figures 2 and SSG performed experiments for Figures 3, 4 and 6. LSF and SSG equally contributed data in Figure 1. JS assisted with experiments performed in Figure 2. Pathologists MLS, DLR and JMN blindly scored primary tumors. CW performed statistics. CB performed mass spectrometry. JRM made NM23-H1 constructs and lentiviral stocks. DMK was involved in the experimental planning and design. RP directed the project and wrote the manuscript.
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Fiore, L., Ganguly, S., Sledziona, J. et al. c-Abl and Arg induce cathepsin-mediated lysosomal degradation of the NM23-H1 metastasis suppressor in invasive cancer. Oncogene 33, 4508–4520 (2014). https://doi.org/10.1038/onc.2013.399
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.399
Keywords
This article is cited by
-
Heat-shock protein 90α protects NME1 against degradation and suppresses metastasis of breast cancer
British Journal of Cancer (2023)
-
CTCF and EGR1 suppress breast cancer cell migration through transcriptional control of Nm23-H1
Scientific Reports (2021)
-
The role of lysosomes in cancer development and progression
Cell & Bioscience (2020)
-
Combating acquired resistance to MAPK inhibitors in melanoma by targeting Abl1/2-mediated reactivation of MEK/ERK/MYC signaling
Nature Communications (2020)
-
A rare subpopulation of melanoma cells with low expression of metastasis suppressor NME1 is highly metastatic in vivo
Scientific Reports (2020)