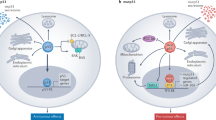
Abstract
MicroRNAs (miRNAs) control cell cycle progression by targeting the transcripts encoding for cyclins, CDKs and CDK inhibitors, such as p27KIP1 (p27). p27 expression is controlled by multiple transcriptional and posttranscriptional mechanisms, including translational inhibition by miR-221/222 and posttranslational regulation by the SCFSKP2 complex. The oncosuppressor activity of miR-340 has been recently characterized in breast, colorectal and osteosarcoma tumor cells. However, the mechanisms underlying miR-340-induced cell growth arrest have not been elucidated. Here, we describe miR-340 as a novel tumor suppressor in non-small cell lung cancer (NSCLC). Starting from the observation that the growth-inhibitory and proapoptotic effects of miR-340 correlate with the accumulation of p27 in lung adenocarcinoma and glioblastoma cells, we have analyzed the functional relationship between miR-340 and p27 expression. miR-340 targets three key negative regulators of p27. The miR-340-mediated inhibition of both Pumilio family RNA-binding proteins (PUM1 and PUM2), required for the miR-221/222 interaction with the p27 3′-UTR, antagonizes the miRNA-dependent downregulation of p27. At the same time, miR-340 induces the stabilization of p27 by targeting SKP2, the key posttranslational regulator of p27. Therefore, miR-340 controls p27 at both translational and posttranslational levels. Accordingly, the inhibition of either PUM1 or SKP2 partially recapitulates the miR-340 effect on cell proliferation and apoptosis. In addition to the effect on tumor cell proliferation, miR-340 also inhibits intercellular adhesion and motility in lung cancer cells. These changes correlate with the miR-340-mediated inhibition of previously validated (MET and ROCK1) and potentially novel (RHOA and CDH1) miR-340 target transcripts. Finally, we show that in a small cohort of NSCLC patients (n=23), representative of all four stages of lung cancer, miR-340 expression inversely correlates with clinical staging, thus suggesting that miR-340 downregulation contributes to the disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Esquela-Kerscher A, Slack FJ . Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259–269.
Croce CM . Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009; 10: 704–714.
Jansson MD, Lund AH . MicroRNA and cancer. Mol Oncol 2012; 6: 590–610.
van Kouwenhove M, Kedde M, Agami R . MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer 2011; 11: 644–656.
Aqeilan RI, Calin GA, Croce CM . miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 2009; 17: 215–220.
Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, Hasan L et al. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res 2009; 69: 5553–5559.
Hermeking H . The miR-34 family in cancer and apoptosis. Cell Death Differ 2010; 17: 193–199.
Petrocca F . E2F1-regulated microRNAs impair TGFβ-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell 2008; 13: 272–286.
le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A et al. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J 2007; 26: 3699–3708.
Medina R, Zaidi SK, Liu C-G, Stein JL, vanWijnen AJ, Croce CM et al. MicroRNAs 221 and 222 bypass quiescence and compromise cell survival. Cancer Res 2008; 68: 2773–2780.
Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafre SA et al. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem 2007; 282: 23716–23724.
Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer 2007; 14: 791–798.
Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 2008; 27: 5651–5661.
Nassirpour R, Mehta PP, Baxi SM, Yin M-J . miR-221 promotes tumorigenesis in human Triple negative breast cancer cells. PLoS ONE 2013; 8: e62170.
Garofalo M, Leva GD, Romano G, Nuovo G, Suh S-S, Ngankeu A et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009; 16: 498–509.
Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JAF, Elkon R, Agami R . A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol 2010; 12: 1014–1020.
Chu IM, Hengst L, Slingerland JM . The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 2008; 8: 253–267.
Frescas D, Pagano M . Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 2008; 8: 438–449.
Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell 2004; 6: 661–672.
Lin P-Y, Yu S-L, Yang P-C . MicroRNA in lung cancer. Br J Cancer 2010; 103: 1144–1148.
Kasinski AL, Slack FJ . Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 2011; 11: 849–864.
Gennarino VA, D'Angelo G, Dharmalingam G, Fernandez S, Russolillo G, Sanges R et al. Identification of microRNA-regulated gene networks by expression analysis of target genes. Genome Res 2012; 22: 1163–1172.
Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 2007; 67: 2456–2468.
Dong H, Luo L, Hong S, Siu H, Xiao Y, Jin L et al. Integrated analysis of mutations, miRNA and mRNA expression in glioblastoma. BMC Syst Biol 2010; 4: 163.
le Sage C, Nagel R, Agami R . Diverse ways to control p27Kip1 function: miRNAs come into play. Cell Cycle 2007; 6: 2742–2749.
Zhang C . PUMA is a novel target of miR-221/222 in human epithelial cancers. Int J Oncol 2010; 37: 1–6.
Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R . Fast and effective prediction of microRNA/target duplexes. RNA 2004; 10: 1507–1517.
Goswami S, Tarapore RS, Teslaa JJ, Grinblat Y, Setaluri V, Spiegelman VS . MicroRNA-340-mediated degradation of microphthalmia-associated transcription factor mRNA is inhibited by the coding region determinant-binding protein. J Biol Chem 2010; 285: 20532–20540.
Wu Z-S, Wu Q, Wang C-Q, Wang X-N, Huang J, Zhao J-J et al. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer 2011; 117: 2842–2852.
Zhou X, Wei M, Wang W . MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem Biophys Res Commun 2013; 437: 653–658.
Das S, Bryan K, Buckley PG, Piskareva O, Bray IM, Foley N et al. Modulation of neuroblastoma disease pathogenesis by an extensive network of epigenetically regulated microRNAs. Oncogene 2012; 32: 2927–2936.
Hu Y . miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep 2012; 28: 1346–1352.
Wang X, Gorospe M, Huang Y, Holbrook NJ . p27Kip1 overexpression causes apoptotic death of mammalian cells. Oncogene 1997; 15: 2991–2997.
Galgano A, Forrer M, Jaskiewicz L, Kanitz A, Zavolan M, Gerber AP . Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS ONE 2008; 3: e3164.
Miles WO, Tschöp K, Herr A, Ji J-Y, Dyson NJ . Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev 2012; 26: 356–368.
Huang Y-H, Wu C-C, Chou C-K, Huang C-YF . A translational regulator, PUM2, promotes both protein stability and kinase activity of Aurora-A. PLoS ONE 2011; 6: e19718.
Hashimoto Y, Akiyama Y, Yuasa Y . Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS ONE 2013; 8: e62589.
Hou J, Aerts J, Hamer den B, van IJcken W, Bakker den M, Riegman P et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS ONE 2010; 5: e10312.
Takanami I . The prognostic value of overexpression of Skp2 mRNA in non-small cell lung cancer. Oncol Rep 2005; 13: 727–731.
Cai H, Lin L, Cai H, Tang M, Wang Z . Combined microRNA-340 and ROCK1 mRNA profiling predicts tumor progression and prognosis in pediatric osteosarcoma. IJMS 2014; 15: 560–573.
Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D'Esposito M et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene 2009; 28: 73–84.
Talotta F, Mega T, Bossis G, Casalino L, Basbous J, Jariel-Encontre I et al. Heterodimerization with Fra-1 cooperates with the ERK pathway to stabilize c-Jun in response to the RAS oncoprotein. Oncogene 2010; 29: 4732–4740.
Acknowledgements
We thank Reuven Agami, Judy Lieberman and Vladimir Spiegelman for expression vectors. We also thank the IGB FACS and Microscopy facilities. This work was supported by the AIRC (Associazione Italiana per la Ricerca sul Cancro) Grant-10489, AICR (Association for International Cancer Research, UK) Grant-08-182 and MERIT Grant-RBNE08YFN3 (MIUR) to Pasquale Verde. Gerolama Condorelli was supported by grants from AIRC (Grant-14046) and Fondazione Berlucchi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Fernandez, S., Risolino, M., Mandia, N. et al. miR-340 inhibits tumor cell proliferation and induces apoptosis by targeting multiple negative regulators of p27 in non-small cell lung cancer. Oncogene 34, 3240–3250 (2015). https://doi.org/10.1038/onc.2014.267
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.267
This article is cited by
-
PUMILIO proteins promote colorectal cancer growth via suppressing p21
Nature Communications (2022)
-
Hsa_circ_001680 affects the proliferation and migration of CRC and mediates its chemoresistance by regulating BMI1 through miR-340
Molecular Cancer (2020)
-
MicroRNA-340-5p inhibits colon cancer cell migration via targeting of RhoA
Scientific Reports (2020)
-
LncRNA MYCNOS facilitates proliferation and invasion in hepatocellular carcinoma by regulating miR-340
Human Cell (2020)
-
Tumor miRNA expression profile is related to vestibular schwannoma growth rate
Acta Neurochirurgica (2020)