Abstract
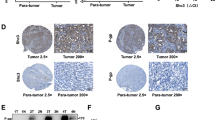
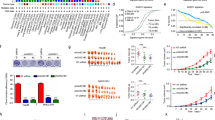
SIRT3 is a class III histone deacetylase that has been implicated in a variety of cancers. The role of SIRT3 in hepatocellular carcinoma (HCC) remains elusive. In this study, we found that SIRT3 expression was frequently repressed in HCC and its downregulation was closely associated with tumor grade and size. Ectopic expression of SIRT3 inhibited cell growth and induced apoptosis in HCC cells, whereas depletion of SIRT3 in immortalized hepatocyte promoted cell growth and decreased epirubicin-induced apoptosis. Mechanistic studies revealed that SIRT3 deacetylated and activated glycogen synthase kinase-3β (GSK-3β), which subsequently induced expression and mitochondrial translocation of the pro-apoptotic protein BCL2-associated X protein (Bax) to promote apoptosis. GSK-3β inhibitor or gene silencing of BAX reversed SIRT3-induced growth inhibition and apoptosis. Furthermore, SIRT3 overexpression also suppressed tumor growth in vivo. Together, this study reveals a role of SIRT3/GSK-3β/Bax signaling pathway in the suppression of HCC growth, and also suggests that targeting this pathway may represent a potential therapeutic approach for HCC treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ding J, Wang H . Multiple interactive factors in hepatocarcinogenesis. Cancer Lett 2014; 346: 17–23.
Hall JA, Dominy JE, Lee Y, Puigserver P . The sirtuin family's role in aging and age-associated pathologies. J Clin Invest 2013; 123: 973–979.
D'Aquila P, Rose G, Panno ML, Passarino G, Bellizzi D . SIRT3 gene expression: a link between inherited mitochondrial DNA variants and oxidative stress. Gene 2012; 497: 323–329.
Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 2011; 20: 487–499.
Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011; 332: 1443–1446.
Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao B et al. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol Cell 2012; 46: 484–494.
Michan S, Sinclair D . Sirtuins in mammals: insights into their biological function. Biochem J 2007; 404: 1–13.
Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB . Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 2005; 123: 437–448.
Hori YS, Kuno A, Hosoda R, Horio Y . Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PloS One 2013; 8: e73875.
Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med 2007; 39: 8–13.
Knight JR, Milner J . SIRT1 metabolism and cancer. Curr Opin Oncol 2012; 24: 68–75.
Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res 2007; 67: 6612–6618.
Elangovan S, Ramachandran S, Venkatesan N, Ananth S, Gnana-Prakasam JP, Martin PM et al. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor alpha in breast cancer. Cancer Res 2011; 71: 6654–6664.
Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res 2011; 71: 4138–4149.
Hida Y, Kubo Y, Murao K, Arase S . Strong expression of a longevity-related protein, SIRT1, in Bowen's disease. Arch Dermatol Res 2007; 299: 103–106.
Giralt A, Villarroya F . SIRT3 a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J 2012; 444: 1–10.
Ashraf N, Zino S, Macintyre A, Kingsmore D, Payne AP, George WD et al. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer 2006; 95: 1056–1061.
Alhazzazi TY, Kamarajan P, Joo N, Huang JY, Verdin E, D'Silva NJ et al. Sirtuin-3 (SIRT3), a novel potential therapeutic target for oral cancer. Cancer 2011; 117: 1670–1678.
Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 2010; 17: 41–52.
Chen J, Chan AW, To KF, Chen W, Zhang Z, Ren J et al. SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3beta/beta-catenin signaling. Hepatology 2013; 57: 2287–2298.
Fabregat I . Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol 2009; 15: 513–520.
Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA et al. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 2004; 24: 9993–10002.
Zhang L, Zhang Y, Xing D . LPLI inhibits apoptosis upstream of Bax translocation via a GSK-3beta-inactivation mechanism. J Cell Physiol 2010; 224: 218–228.
Bijur GN, Jope RS . Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport 2003; 14: 2415–2419.
Byun HO, Jung HJ, Seo YH, Lee YK, Hwang SC, Hwang ES et al. GSK3 inactivation is involved in mitochondrial complex IV defect in transforming growth factor (TGF) beta1-induced senescence. Exp Cell Res 2012; 318: 1808–1819.
Monteserin-Garcia J, Al-Massadi O, Seoane LM, Alvarez CV, Shan B, Stalla J et al. Sirt1 inhibits the transcription factor CREB to regulate pituitary growth hormone synthesis. FASEB J 2013; 27: 1561–1571.
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009; 325: 834–840.
Zhao Y, Yang H, Wang X, Zhang R, Wang C, Guo Z . Sirtuin-3 (SIRT3) expression is associated with overall survival in esophageal cancer. Ann Diagn Pathol 2013; 17: 483–485.
Zhang CZ, Liu L, Cai M, Pan Y, Fu J, Cao Y et al. Low SIRT3 expression correlates with poor differentiation and unfavorable prognosis in primary hepatocellular carcinoma. PloS One 2012; 7: e51703.
Wang JX, Yi Y, Li YW, Cai XY, He HW, Ni XC et al. Down-regulation of sirtuin 3 is associated with poor prognosis in hepatocellular carcinoma after resection. BMC Cancer 2014; 14: 297.
Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL . SIRT3 and cancer: tumor promoter or suppressor? Biochim Biophys Acta 2011; 1816: 80–88.
Marfe G, Tafani M, Indelicato M, Sinibaldi-Salimei P, Reali V, Pucci B et al. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J Cell Biochem 2009; 106: 643–650.
Allison SJ, Milner J . SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways. Cell Cycle 2007; 6: 2669–2677.
Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP . SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 2008; 28: 6384–6401.
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D . Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010; 12: 662–667.
Cheng Y, Ren X, Gowda AS, Shan Y, Zhang L, Yuan YS et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis 2013; 4: e731.
Fabregat I, Roncero C, Fernandez M . Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int 2007; 27: 155–162.
Westphal D, Dewson G, Czabotar PE, Kluck RM . Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta 2011; 1813: 521–531.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81201282), the National Science and Technology Major Project (2013ZX10002002), the Chongqing Natural Science Foundation (cstc2012jjA10047) and the PhD program through the Ministry of Education of China (Program 20125503120004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Song, CL., Tang, H., Ran, LK. et al. Sirtuin 3 inhibits hepatocellular carcinoma growth through the glycogen synthase kinase-3β/BCL2-associated X protein-dependent apoptotic pathway. Oncogene 35, 631–641 (2016). https://doi.org/10.1038/onc.2015.121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.121
This article is cited by
-
Role of SIRT3 in neurological diseases and rehabilitation training
Metabolic Brain Disease (2023)
-
Modulation of SIRT3 expression through CDK4/6 enhances the anti-cancer effect of sorafenib in hepatocellular carcinoma cells
BMC Cancer (2020)
-
Discovery of suppressors of CRMP2 phosphorylation reveals compounds that mimic the behavioral effects of lithium on amphetamine-induced hyperlocomotion
Translational Psychiatry (2020)
-
Dysregulation of histone deacetylases in carcinogenesis and tumor progression: a possible link to apoptosis and autophagy
Cellular and Molecular Life Sciences (2019)
-
Glycogen synthase kinase-3β inhibition promotes lysosome-dependent degradation of c-FLIPL in hepatocellular carcinoma
Cell Death & Disease (2018)