Abstract
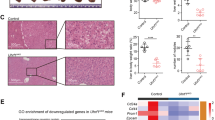
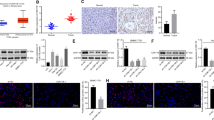
Cancer initiating cells (CICs) are responsible for the unrestrained cell growth and chemoresistance of malignant tumors. Histone demethylation has been shown to be crucial for self-renewal/differentiation of stem cells, but it remains elusive whether lysine-specific demethylase 1 (LSD1) regulates the stemness properties of CICs. Here we report that the abundant expression of leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) is associated with the progression of hepatocellular carcinoma (HCC). Lgr5+ HCC cells behave similarly to CICs and are highly tumorigenic and resistant to chemotherapeutic agents. Importantly, Lgr5+ cells express higher levels of LSD1, which in turn regulates Lgr5 expression and promotes the self-renewal and drug resistance of Lgr5+ CICs. Mechanistically, LSD1 promotes β-catenin activation by inhibiting the expression of several suppressors of β-catenin signaling, especially Prickle1 and APC in Lgr5+ CICs, by directly regulating the levels of mono- and di-methylation of histone H3 lysine-4 at the promoters of these genes. Furthermore, LSD1-associated activation of the β-catenin signaling is essential for maintaining the activity of Lgr5+ CICs. Together, our findings unravel the LSD1/Prickle1/APC/β-catenin signaling axis as a novel molecular circuit regulating the stemness and chemoresistance of hepatic Lgr5+ CICs and provide potential targets to improve chemotherapeutic efficacies against HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
11 June 2015
This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue.
References
Yamashita T, Wang XW . Cancer stem cells in the development of liver cancer. J Clin Invest 2013; 123: 1911–1918.
Meacham CE, Morrison SJ . Tumour heterogeneity and cancer cell plasticity. Nature 2013; 501: 328–337.
Wang Z, Wang B, Shi Y, Xu C, Xiao HL, Ma LN et al. Oncogenic miR-20a and miR-106a enhance the invasiveness of human glioma stem cells by directly targeting TIMP-2. Oncogene 2015; 34: 1407–1419.
Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res 2014; 74: 5746–5757.
Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013; 494: 247–250.
Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R et al. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology 2007; 133: 1579–1591.
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res 2008; 68: 4287–4295.
Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010; 6: 25–36.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007.
Qiu W, Wang X, Buchanan M, He K, Sharma R, Zhang L et al. ADAR1 is essential for intestinal homeostasis and stem cell maintenance. Cell Death Dis 2013; 4: e599.
Hirsch D, Barker N, McNeil N, Hu Y, Camps J, McKinnon K et al. LGR5 positivity defines stem-like cells in colorectal cancer. Carcinogenesis 2014; 35: 849–858.
Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 2012; 337: 730–735.
Kemper K, Prasetyanti PR, De Lau W, Rodermond H, Clevers H, Medema JP . Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells 2012; 30: 2378–2386.
Fukuma M, Tanese K, Effendi K, Yamazaki K, Masugi Y, Suda M et al. Leucine-rich repeat-containing G protein-coupled receptor 5 regulates epithelial cell phenotype and survival of hepatocellular carcinoma cells. Exp Cell Res 2013; 319: 113–121.
Greer EL, Shi Y . Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 2012; 13: 343–357.
Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 2009; 138: 660–672.
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004; 119: 941–953.
Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 2005; 437: 436–439.
Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ et al. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol 2011; 13: 652–659.
Wang J, Lu F, Ren Q, Sun H, Xu Z, Lan R et al. Novel histone demethylase LSD1 inhibitors selectively target cancer cells with pluripotent stem cell properties. Cancer Res 2011; 71: 7238–7249.
Harris WJ, Huang X, Lynch JT, Spencer GJ, Hitchin JR, Li Y et al. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell 2012; 21: 473–487.
Zhao ZK, Dong P, Gu J, Chen L, Zhuang M, Lu WJ et al. Overexpression of LSD1 in hepatocellular carcinoma: a latent target for the diagnosis and therapy of hepatoma. Tumour Biol 2013; 34: 173–180.
Yu Y, Wang B, Zhang K, Lei Z, Guo Y, Xiao H et al. High expression of lysine-specific demethylase 1 correlates with poor prognosis of patients with esophageal squamous cell carcinoma. Biochem Biophys Res Commun 2013; 437: 192–198.
Hayami S, Kelly JD, Cho HS, Yoshimatsu M, Unoki M, Tsunoda T et al. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer 2011; 128: 574–586.
Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 2014; 20: 29–36.
Shan J, Shen J, Liu L, Xia F, Xu C, Duan G et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology 2012; 56: 1004–1014.
Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO . CD24+ liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 2011; 9: 50–63.
Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest 2010; 120: 3326–3339.
Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009; 136: 1012–1024.
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007; 132: 2542–2556.
O'Brien CA, Kreso A, Ryan P, Hermans KG, Gibson L, Wang Y et al. ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21. Cancer Cell 2012; 21: 777–792.
Chan DW, Chan CY, Yam JW, Ching YP, Ng IO . Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology 2006; 131: 1218–1227.
Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013; 500: 222–226.
Wang B, Liu J, Ma LN, Xiao HL, Wang YZ, Li Y et al. Chimeric 5/35 adenovirus-mediated Dickkopf-1 overexpression suppressed tumorigenicity of CD44+ gastric cancer cells via attenuating Wnt signaling. J Gastroenterol 2013; 48: 798–808.
Plaks V, Brenot A, Lawson DA, Linnemann JR, Van Kappel EC, Wong KC et al. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep 2013; 3: 70–78.
Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY . Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 2013; 495: 241–245.
Gil-Sanchis C, Cervello I, Mas A, Faus A, Pellicer A, Simon C . Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) as a putative human endometrial stem cell marker. Mol Hum Reprod 2013; 19: 407–414.
Xi HQ, Cai AZ, Wu XS, Cui JX, Shen WS, Bian SB et al. Leucine-rich repeat-containing G-protein-coupled receptor 5 is associated with invasion, metastasis, and could be a potential therapeutic target in human gastric cancer. Br J Cancer 2014; 110: 2011–2020.
Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011; 43: 34–41.
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008; 13: 153–166.
Yamashita T, Honda M, Nakamoto Y, Baba M, Nio K, Hara Y et al. Discrete nature of EpCAM+ and CD90+ cancer stem cells in human hepatocellular carcinoma. Hepatology 2013; 57: 1484–1497.
Ding J, Zhang ZM, Xia Y, Liao GQ, Pan Y, Liu S et al. LSD1-mediated epigenetic modification contributes to proliferation and metastasis of colon cancer. Br J Cancer 2013; 109: 994–1003.
Qin Y, Zhu W, Xu W, Zhang B, Shi S, Ji S et al. LSD1 sustains pancreatic cancer growth via maintaining HIF1alpha-dependent glycolytic process. Cancer Lett 2014; 347: 225–232.
Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 2014; 157: 580–594.
Wu Y, Wang Y, Yang XH, Kang T, Zhao Y, Wang C et al. The deubiquitinase USP28 stabilizes LSD1 and confers stem-cell-like traits to breast cancer cells. Cell Rep 2013; 5: 224–236.
Schenk T, Chen WC, Gollner S, Howell L, Jin L, Hebestreit K et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med 2012; 18: 605–611.
Zhang X, Lu F, Wang J, Yin F, Xu Z, Qi D et al. Pluripotent stem cell protein Sox2 confers sensitivity to LSD1 inhibition in cancer cells. Cell Rep 2013; 5: 445–457.
Huang Z, Li S, Song W, Li X, Li Q, Zhang Z et al. Lysine-specific demethylase 1 (LSD1/KDM1A) contributes to colorectal tumorigenesis via activation of the Wnt/Beta-catenin pathway by down-regulating Dickkopf-1 (DKK1). PLoS One 2013; 8: e70077.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390.
Wang B, Yu SC, Jiang JY, Porter GW, Zhao LT, Wang Z et al. An inhibitor of arachidonate 5-lipoxygenase, Nordy, induces differentiation and inhibits self-renewal of glioma stem-like cells. Stem Cell Rev 2011; 7: 458–470.
Acknowledgements
We are indebted to Prof Xiang Xu (Research Institute of Surgery, Daping Hospital, Third Military Medical University, Chongqing, China) for his technical assistance. We are grateful to Miss Chunhua Quan, Ya Li and Qing Li (Department of Gastroenterology, Institute of Surgery Research, Daping Hospital, Third Military Medical University, Chongqing, China) for their help in collecting clinical data. This work was supported by the grants from the National Natural Science Foundation of China (NSFC Nos. 81472294, 81201949, and 81372558), the Natural Science Foundation Project of CQ CSTC (No. CSTC2012JJA10124), and the Science Foundation of Third Military Medical University (No. 2012XJQ22).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lei, ZJ., Wang, J., Xiao, HL. et al. Lysine-specific demethylase 1 promotes the stemness and chemoresistance of Lgr5+ liver cancer initiating cells by suppressing negative regulators of β-catenin signaling. Oncogene 34, 3188–3198 (2015). https://doi.org/10.1038/onc.2015.129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.129
This article is cited by
-
Comprehensive Review on the Effect of Stem Cells in Cancer Progression
Current Tissue Microenvironment Reports (2024)
-
Aberrant RNA m6A modification in gastrointestinal malignancies: versatile regulators of cancer hallmarks and novel therapeutic opportunities
Cell Death & Disease (2023)
-
Targeting lysine-specific demethylase 1 (KDM1A/LSD1) impairs colorectal cancer tumorigenesis by affecting cancer cells stemness, motility, and differentiation
Cell Death Discovery (2023)
-
E3 ubiquitin ligases in cancer stem cells: key regulators of cancer hallmarks and novel therapeutic opportunities
Cellular Oncology (2023)
-
Histone methylation in pre-cancerous liver diseases and hepatocellular carcinoma: recent overview
Clinical and Translational Oncology (2023)