Abstract
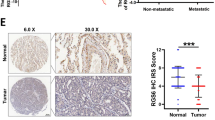
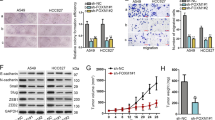
TIF1γ is a novel regulator of transforming growth factor (TGF)-β/Smad signaling. Our previous studies show that dysregulated expression of transcriptional intermediary factor 1 γ (TIF1γ) and abnormal TGF-β/Smad signaling are implicated in non-small-cell lung cancer (NSCLC) separately. However, how TIF1γ contributes to NSCLC by controlling TGF-β/Smad signaling is poorly understood. Here, we investigated the mechanistic role of TIF1γ in TGF-β-induced epithelial–mesenchymal transition (EMT), as well as a link between TIF1γ and SOX2 in NSCLC. We show that TIF1γ is a downstream target of SOX2 in NSCLC cells. SOX2 overexpression negatively regulated TIF1γ promoter activity and thereby attenuated TIF1γ mRNA and protein expression levels; SOX2 knockdown significantly enhanced TIF1γ promoter activity and augmented TIF1γ expression. Moreover, TIF1γ mRNA expression was downregulated in human NSCLC tissues and negatively correlated with SOX2 protein, which was upregulated in NSCLC tissues. Importantly, knockdown of TIF1γ or SOX2 overexpression augmented SMAD4 (human Mad (mothers against decapentaplegic)-related homologous protein 4)-dependent transcriptional responses, and enhanced TGF-β-induced EMT and human NSCLC cell invasion; knockdown of SOX2 impaired TGF-β-induced EMT and NSCLC cell invasion. In an in vivo model of metastasis, knockdown of TIF1γ promotes NSCLC cell metastasis. In addition, our data suggested that TIF1γ inhibited TGF-β-induced EMT through competing with SMAD4 in NSCLC cells. Taken together, our findings reveal a new mechanism by which SOX2-mediated transcription repression of TIF1γ promotes TGF-β-induced EMT in NSCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lei Z, Liu RY, Zhao J, Liu Z, Jiang X, You W et al. TGFBR1 haplotypes and risk of non-small-cell lung cancer. Cancer Res 2009; 69: 7046–7052.
Feng J, Zhang X, Zhu H, Wang X, Ni S, Huang J . High expression of FoxP1 is associated with improved survival in patients with non-small cell lung cancer. Am J Clin Pathol 2012; 138: 230–235.
Gupta GP, Massague J. . Cancer metastasis: building a framework. Cell 2006; 127: 679–695.
Meulmeester E, Ten Dijke P . The dynamic roles of TGF-beta in cancer. J Pathol 2011; 223: 205–218.
Massague J, Blain SW, Lo RS . TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 2000; 103: 295–309.
Jeon HS, Jen J . TGF-beta signaling and the role of inhibitory Smads in non-small cell lung cancer. J Thorac Oncol 2010; 5: 417–419.
Massague J, Seoane J, Wotton D . Smad transcription factors. Genes Dev 2005; 19: 2783–2810.
Park C, Kim WS, Choi Y, Kim H, Park K . Effects of transforming growth factor beta (TGF-beta) receptor on lung carcinogenesis. Lung Cancer 2002; 38: 143–147.
Zhao J, Liu Z, Li W, Liu X, Chen XF, Zhang HT . Infrequently methylated event at sites -362 to -142 in the promoter of TGF beta R1 gene in non-small cell lung cancer. J Cancer Res Clin Oncol 2008; 134: 919–925.
Zhang HT, Chen XF, Wang MH, Wang JC, Qi QY, Zhang RM et al. Defective expression of transforming growth factor beta receptor type II is associated with CpG methylated promoter in primary non-small cell lung cancer. Clin Cancer Res 2004; 10: 2359–2367.
Lei Z, Xu G, Wang L, Yang H, Liu X, Zhao J et al. MiR-142-3p represses TGF-beta-induced growth inhibition through repression of TGFbetaR1 in non-small cell lung cancer. FASEB J 2014; 28: 2696–2704.
Massague J . TGFbeta in Cancer. Cell 2008; 134: 215–230.
Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell 2011; 22: 1686–1698.
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z et al. JAK/STAT3 signaling is required for TGF-beta-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol 2014; 44: 1643–1651.
Kang Y, Massague J . Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 2004; 118: 277–279.
Thiery JP . Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L et al. TGF-beta: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst 2014; 106: djt369.
Venturini L, You J, Stadler M, Galien R, Lallemand V, Koken MH et al. TIF1 gamma, a novel member of the transcriptional intermediary factor 1 family. Oncogene 1999; 18: 1209–1217.
Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, Rugge M et al. Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell 2005; 121: 87–99.
He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J . Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell 2006; 125: 929–941.
Aucagne R, Droin N, Paggetti J, Lagrange B, Largeot A, Hammann A et al. Transcription intermediary factor 1gamma is a tumor suppressor in mouse and human chronic myelomonocytic leukemia. J Clin Invest 2011; 121: 2361–2370.
Vincent DF, Yan KP, Treilleux I, Gay F, Arfi V, Kaniewski B et al. Inactivation of TIF1gamma cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet 2009; 5: e1000575.
Wang L, Lei Z, Liu X, Liu R, Zhang H . [Association of mutation and methylation in the promoter region of TIF1gamma with non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi 2013; 16: 227–232.
Fattet L, Ay AS, Bonneau B, Jallades L, Mikaelian I, Treilleux I et al. TIF1gamma requires sumoylation to exert its repressive activity on TGFbeta signaling. J Cell Sci 2013; 126: 3713–3723.
Hesling C, Fattet L, Teyre G, Jury D, Gonzalo P, Lopez J et al. Antagonistic regulation of EMT by TIF1gamma and Smad4 in mammary epithelial cells. EMBO Rep 2011; 12: 665–672.
Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R . Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol 2008; 317: 296–309.
Que J, Luo X, Schwartz RJ, Hogan BL . Multiple roles for Sox2 in the developing and adult mouse trachea. Development 2009; 136: 1899–1907.
Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B . Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol 2007; 39: 2195–2214.
Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R . Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 2003; 17: 126–140.
Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A et al. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal 2013; 25: 1264–1271.
Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS . MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 2009; 137: 647–658.
Xu W, Wei Y, Tan Y, Cheng SY, Wu J . [The expression and significance of stem cell transcription factor Sox2 in lung carcinoma]. Zhongguo Fei Ai Za Zhi 2013; 16: 591–595.
Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, Murase M et al. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest 2011; 91: 1796–1804.
Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP . The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 2014; 25: 139–151.
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/beta-catenin signal network. Cancer Lett 2013; 336: 379–389.
Han X, Fang X, Lou X, Hua D, Ding W, Foltz G et al. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PLoS One 2012; 7: e41335.
Pirozzi G, Tirino V, Camerlingo R, Franco R, La Rocca A, Liguori E et al. Epithelial to mesenchymal transition by TGFbeta-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One 2011; 6: e21548.
Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D et al. Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int J Cancer 1998; 77: 7–12.
Bennett WP, el-Deiry WS, Rush WL, Guinee DG Jr., Freedman AN, Caporaso NE et al. p21waf1/cip1 and transforming growth factor beta 1 protein expression correlate with survival in non-small cell lung cancer. Clin Cancer Res 1998; 4: 1499–1506.
Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G et al. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res 2006; 66: 2202–2209.
Akhurst RJ, Balmain A . Genetic events and the role of TGF beta in epithelial tumour progression. J Pathol 1999; 187: 82–90.
Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest 2005; 115: 1714–1723.
Xue J, Lin X, Chiu WT, Chen YH, Yu G, Liu M et al. Sustained activation of SMAD3/SMAD4 by FOXM1 promotes TGF-beta-dependent cancer metastasis. J Clin Invest 2014; 124: 564–579.
Zhou CH, Ye LP, Ye SX, Li Y, Zhang XY, Xu XY et al. Clinical significance of SOX9 in human non-small cell lung cancer progression and overall patient survival. J Exp Clin Cancer Res 2012; 31: 18.
Szymanowska-Narloch A, Jassem E, Skrzypski M, Muley T, Meister M, Dienemann H et al. Molecular profiles of non-small cell lung cancers in cigarette smoking and never-smoking patients. Adv Med Sci 2013; 58: 196–206.
Fang WT, Fan CC, Li SM, Jang TH, Lin HP, Shih NY et al. Downregulation of a putative tumor suppressor BMP4 by SOX2 promotes growth of lung squamous cell carcinoma. Int J Cancer 2014; 135: 809–819.
Qian Q, Shi X, Lei Z, Zhan L, Liu RY, Zhao J et al. Methylated +58CpG site decreases DCN mRNA expression and enhances TGF-beta/Smad signaling in NSCLC cells with high metastatic potential. Int J Oncol 2014; 44: 874–882.
Acknowledgements
We are grateful for participation and cooperation from the patients with NSCLC. This work was supported in part by the grants from National Natural Science Foundation of China (81372277, 81171894 and 81201575), Jiangsu Province’s Key Provincial Talents Program (RC2011106), ‘333’ Project of Jiangsu Province Government, Graduate Innovation Project of Jiangsu Province (CXZZ13_0830), Soochow Scholar Project of Soochow University, Suzhou Key Laboratory for Molecular Cancer Genetics (SZS201209) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, L., Yang, H., Lei, Z. et al. Repression of TIF1γ by SOX2 promotes TGF-β-induced epithelial–mesenchymal transition in non-small-cell lung cancer. Oncogene 35, 867–877 (2016). https://doi.org/10.1038/onc.2015.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.141
This article is cited by
-
Downregulation of PTPRT elevates the expression of survivin and promotes the proliferation, migration, and invasion of lung adenocarcinoma
BMC Cancer (2024)
-
Advances of autoimmune rheumatic diseases related to malignant tumors
Inflammation Research (2023)
-
Reciprocal FGF19-GLI2 signaling induces epithelial-to-mesenchymal transition to promote lung squamous cell carcinoma metastasis
Cellular Oncology (2023)
-
Development of dermatomyositis after anti-transcriptional intermediary factor 1-γ antibody seroconversion during treatment for small cell lung cancer
BMC Pulmonary Medicine (2022)
-
RGS6 suppresses TGF-β-induced epithelial–mesenchymal transition in non-small cell lung cancers via a novel mechanism dependent on its interaction with SMAD4
Cell Death & Disease (2022)