Abstract
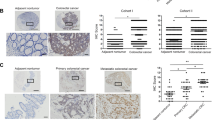
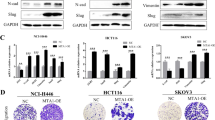
Dysregulation of the Hippo pathway occurs in a variety of cancers and often correlates with a poor prognosis. To further explore the potential role of Hippo pathway dysregulation in tumor development and progression, we investigated its downstream transcription factor TEAD4 in colorectal cancer (CRC). Increased expression and nuclear localization of TEAD4 were found in a significant portion of CRC tissues, in association with metastasis and a poor prognosis. In CRC cells, TEAD4 knockdown induced the mesenchymal–epithelial transition and decreased cell mobility in vitro and metastasis in vivo. Microarray analysis revealed that TEAD4 promoted cell adhesion and upregulated the epithelial–mesenchymal transition-related transcriptome in CRC cells. Vimentin was identified as a new direct target gene mediating TEAD4 function in CRC cells, whereby forced vimentin expression markedly reversed TEAD4-knockdown-induced cell morphological changes and decreased mobility. Interestingly, rescued expression of both WT TEAD4 and a Y429H mutant can reverse the mesenchymal–epithelial transition and increase vimentin expression, cell mobility and metastatic potential in TEAD4-knockdown CRC cells. The discrepant expression of YAP and TEAD4 in CRC tissues, the rescue ability of TEAD4 mutant defect in YAP binding and no effect on vimentin expression by YAP knockdown in CRC cells, all implicated a YAP-independent manner of TEAD4 function in CRC. Furthermore, vimentin positively correlated and CDH1 reversely correlated with the level of TEAD4 in CRC tissues and xenograft tumors. Our results suggest that TEAD4 nuclear expression can serve as a biomarker for CRC progression and poor prognosis. The transcription factor TEAD4 regulates a pro-metastasis transcription program in a YAP-independent manner in CRC, thus providing a novel mechanism of TEAD4 transcriptional regulation and its oncogenic role in CRC, independently of the Hippo pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Markowitz SD, Bertagnolli MM . Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med 2009; 361: 2449–2460.
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier A-M . Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006; 244: 254.
Harvey KF, Zhang X, Thomas DM . The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257.
Lamar JM, Stern P, Liu H, Schindler JW, Jiang Z-G, Hynes RO . The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA 2012; 109: E2441–E2450.
Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee S-J, Anders RA et al. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev 2012; 26: 1300–1305.
Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337.
Huang DW, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 2008; 4: 44–57.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le Q-T et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006; 440: 1222–1226.
Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET . Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res 2005; 65: 7554–7560.
Leung W, Ching A, Chan A, Poon T, Mian H, Wong et al. A novel interplay between oncogenic PFTK1 protein kinase and tumor suppressor TAGLN2 in the control of liver cancer cell motility. Oncogene 2011; 30: 4464–4475.
Kobayashi H, Sagara J, Kurita H, Morifuji M, Ohishi M, Kurashina K et al. Clinical significance of cellular distribution of moesin in patients with oral squamous cell carcinoma. Clin Cancer Res 2004; 10: 572–580.
Larkin SB, Farrance I, Ordahl CP . Flanking sequences modulate the cell specificity of M-CAT elements. Mol Cell Biol 1996; 16: 3742–3755.
Chung B-M, Rotty JD, Coulombe PA . Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol 2013; 25: 600–612.
Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P et al. Transactivation of vimentin by β-catenin in human breast cancer cells. Cancer Res 2003; 63: 2658–2664.
Felipe De Sousa EM, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med 2013; 19: 614–618.
Kahlert C, Lahes S, Radhakrishnan P, Dutta S, Mogler C, Herpel E et al. Overexpression of ZEB2 at the invasion front of colorectal cancer is an independent prognostic marker and regulates tumor invasion in vitro. Clin Cancer Res 2011; 17: 7654–7663.
Chen Z, Friedrich GA, Soriano P . Transcriptional enhancer factor 1 disruption by a retroviral gene trap leads to heart defects and embryonic lethality in mice. Genes Dev 1994; 8: 2293–2301.
Kaneko KJ, Kohn MJ, Liu C, DePamphilis ML . Transcription factor TEAD2 is involved in neural tube closure. Genesis 2007; 45: 577–587.
Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML et al. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development 2007; 134: 3827–3836.
Hucl T, Brody JR, Gallmeier E, Iacobuzio-Donahue CA, Farrance IK, Kern SE . High cancer-specific expression of mesothelin (MSLN) is attributable to an upstream enhancer containing a transcription enhancer factor–dependent MCAT motif. Cancer Res 2007; 67: 9055–9065.
Kaneko KJ, DePamphilis ML . TEAD4 establishes the energy homeostasis essential for blastocoel formation. Development 2013; 140: 3680–3690.
Home P, Saha B, Ray S, Dutta D, Gunewardena S, Yoo B et al. Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci 2012; 109: 7362–7367.
Hirate Y, Cockburn K, Rossant J, Sasaki H . Tead4 is constitutively nuclear, while nuclear vs. cytoplasmic Yap distribution is regulated in preimplantation mouse embryos. Proc Natl Acad Sci USA 2012; 109: E3389–E3390.
Lim B, Park J-L, Kim H-J, Park Y-K, Kim J-H, Sohn HA et al. Integrative genomics analysis reveals the multilevel dysregulation and oncogenic characteristics of TEAD4 in gastric cancer. Carcinogenesis 2014; 35: 1020–1027.
Wu S, Liu Y, Zheng Y, Dong J, Pan D . The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 2008; 14: 388–398.
Zhao B, Ye X, Yu J, Li L, Li W, Li S et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008; 22: 1962–1971.
Zhang H, Liu C-Y, Zha Z-Y, Zhao B, Yao J, Zhao S et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem 2009; 284: 13355–13362.
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One 2013; 8: e65539.
Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 2012; 151: 1457–1473.
Xiao JH, Davidson I, Matthes H, Garnier J-M, Chambon P . Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell 1991; 65: 551–568.
Pobbati AV, Chan SW, Lee I, Song H, Hong W . Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure 2012; 20: 1135–1140.
Belandia B, Parker MG . Functional interaction between the p160 coactivator proteins and the transcriptional enhancer factor family of transcription factors. J Biol Chem 2000; 275: 30801–30805.
Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer 2014; 110: 450–458.
Zhong XY, Zhang LH, Jia SQ, Shi T, Niu ZJ, Du H et al. Positive association of up‐regulated Cripto‐1 and down‐regulated E‐cadherin with tumour progression and poor prognosis in gastric cancer. Histopathology 2008; 52: 560–568.
Jackstadt R, Röh S, Neumann J, Jung P, Hoffmann R, Horst D et al. AP4 is a mediator of epithelial–mesenchymal transition and metastasis in colorectal cancer. J Exp Med 2013; 210: 1331–1350.
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant no. 81372636 and No. 81302089), the National High Technology Research and Development Program of China (863 Program) (grant no. SQ2014SFOZD00314), the Shanghai Excellent Young Teachers Program (grant no. ZZjdyx13074).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, G., Yang, Y. et al. Increased TEAD4 expression and nuclear localization in colorectal cancer promote epithelial–mesenchymal transition and metastasis in a YAP-independent manner. Oncogene 35, 2789–2800 (2016). https://doi.org/10.1038/onc.2015.342
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.342
This article is cited by
-
TEAD4 functions as a prognostic biomarker and triggers EMT via PI3K/AKT pathway in bladder cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
CK2-induced cooperation of HHEX with the YAP-TEAD4 complex promotes colorectal tumorigenesis
Nature Communications (2022)
-
TEAD4 overexpression suppresses thyroid cancer progression and metastasis in vitro by modulating Wnt signaling
Journal of Biosciences (2022)
-
Arginine is an epigenetic regulator targeting TEAD4 to modulate OXPHOS in prostate cancer cells
Nature Communications (2021)
-
The clinical relevance of the Hippo pathway in pancreatic ductal adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2021)