Abstract
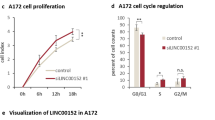
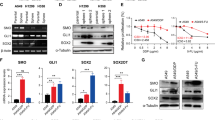
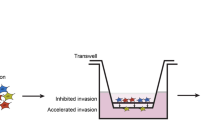
Glioblastoma is the most aggressive primary brain tumor in adults and due to the invasive nature cannot be completely removed. The WNT inhibitory factor 1 (WIF1), a secreted inhibitor of WNTs, is systematically downregulated in glioblastoma and acts as strong tumor suppressor. The aim of this study was the dissection of WIF1-associated tumor-suppressing effects mediated by canonical and non-canonical WNT signaling. We found that WIF1 besides inhibiting the canonical WNT pathway selectively downregulates the WNT/calcium pathway associated with significant reduction of p38-MAPK (p38-mitogen-activated protein kinase) phosphorylation. Knockdown of WNT5A, the only WNT ligand overexpressed in glioblastoma, phenocopied this inhibitory effect. WIF1 expression inhibited cell migration in vitro and in an orthotopic brain tumor model, in accordance with the known regulatory function of the WNT/Ca2+ pathway on migration and invasion. In search of a mediator for this function differential gene expression profiles of WIF1-expressing cells were performed. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), a long non-coding RNA and key positive regulator of invasion, emerged as the top downregulated gene. Indeed, knockdown of MALAT1 reduced migration in glioblastoma cells, without effect on proliferation. Hence, loss of WIF1 enhances the migratory potential of glioblastoma through WNT5A that activates the WNT/Ca2+ pathway and MALAT1. These data suggest the involvement of canonical and non-canonical WNT pathways in glioblastoma promoting key features associated with this deadly disease, proliferation on one hand and invasion on the other. Successful targeting will require a dual strategy affecting both canonical and non-canonical WNT pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996.
Wick W, Stupp R, Beule AC, Bromberg J, Wick A, Ernemann U et al. A novel tool to analyze MRI recurrence patterns in glioblastoma. Neuro Oncol 2008; 10: 1019–1024.
Moon RT, Brown JD, Torres M . WNTs modulate cell fate and behavior during vertebrate development. Trends Genet 1997; 13: 157–162.
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002; 111: 241–250.
Reya T, Clevers H . Wnt signalling in stem cells and cancer. Nature 2005; 434: 843–850.
Boerboom D, White LD, Dalle S, Courty J, Richards JS . Dominant-stable beta-catenin expression causes cell fate alterations and Wnt signalling antagonist expression in a murine granulosa cell tumor model. Cancer Res 2006; 66: 1964–1973.
Voloshanenko O, Erdmann G, Dubash TD, Augustin I, Metzig M, Moffa G et al. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat Commun 2013; 4: 2610.
Polakis P . Wnt signalling in cancer. Cold Spring Harb Perspect Biol 2012; 4: pii: a008052.
Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y et al. Wnt5a suppresses colon cancer by inhibiting cell proliferation and epithelial-mesenchymal transition. J Cell Physiol 2014; 229: 1908–1917.
Lambiv WL, Vassallo I, Delorenzi M, Shay T, Diserens AC, Misra A et al. The Wnt inhibitory factor 1 (WIF1) is targeted in glioblastoma and has a tumor suppressing function potentially by induction of senescence. Neuro Oncol 2011; 13: 736–747.
Gotze S, Wolter M, Reifenberger G, Muller O, Sievers S . Frequent promoter hypermethylation of Wnt pathway inhibitor genes in malignant astrocytic gliomas. Int J Cancer 2010; 126: 2584–2593.
Morris LG, Kaufman AM, Gong Y, Ramaswami D, Walsh LA, Turcan S et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat Genet 2013; 45: 253–261.
Gong A, Huang S . FoxM1 and Wnt/beta-catenin signalling in glioma stem cells. Cancer Res 2012; 72: 5658–5662.
Kamino M, Kishida M, Kibe T, Ikoma K, Iijima M, Hirano H et al. Wnt-5a signalling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP-2. Cancer Sci 2011; 102: 540–548.
Yu JM, Jun ES, Jung JS, Suh SY, Han JY, Kim JY et al. Role of Wnt5a in the proliferation of human glioblastoma cells. Cancer Lett 2007; 257: 172–181.
Kawano Y, Kypta R . Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003; 116: 2627–2634.
Enomoto M, Hayakawa S, Itsukushima S, Ren DY, Matsuo M, Tamada K et al. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signalling. Oncogene 2009; 28: 3197–3208.
Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 2006; 175: 555–562.
Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 2003; 8: 645–654.
Semenov MV, Habas R, Macdonald BT, He X . SnapShot: noncanonical Wnt signalling pathways. Cell 2007; 131: 1378.
De A . Wnt/Ca2+ signalling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai) 2011; 43: 745–756.
Ma L, Wang HY . Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J Biol Chem 2007; 282: 28980–28990.
Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF et al. Stem cell-related "self-renewal" signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol 2008; 26: 3015–3024.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR et al. The somatic genomic landscape of glioblastoma. Cell 2013; 155: 462–477.
Li G, Zhang H, Wan X, Yang X, Zhu C, Wang A et al. Long noncoding RNA plays a key role in metastasis and prognosis of hepatocellular carcinoma. Biomed Res Int 2014; 2014: 780521.
Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett 2010; 584: 4575–4580.
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 2010; 39: 925–938.
Xu C, Yang M, Tian J, Wang X, Li Z . MALAT-1: a long non-coding RNA and its important 3' end functional motif in colorectal cancer metastasis. Int J Oncol 2011; 39: 169–175.
Yamamoto H, Oue N, Sato A, Hasegawa Y, Matsubara A, Yasui W et al. Wnt5a signalling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 2010; 29: 2036–2046.
Bitler BG, Nicodemus JP, Li H, Cai Q, Wu H, Hua X et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res 2011; 71: 6184–6194.
Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 2003; 4: 349–360.
Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC et al. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signalling, and is frequently methylated in colorectal cancer. Clin Cancer Res 2008; 14: 55–61.
Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G . Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 2005; 24: 2144–2154.
Habu M, Koyama H, Kishida M, Kamino M, Iijima M, Fuchigami T et al. Ryk is essential for Wnt-5a-dependent invasiveness in human glioma. J Biochem 2014; 156: 29–38.
Pulvirenti T, Van Der Heijden M, Droms LA, Huse JT, Tabar V, Hall A . Dishevelled 2 signalling promotes self-renewal and tumorigenicity in human gliomas. Cancer Res 2011; 71: 7280–7290.
Ono K, Han J . The p38 signal transduction pathway: activation and function. Cell Signal 2000; 12: 1–13.
Zhang Z, Lv J, Lei X, Li S, Zhang Y, Meng L et al. Baicalein reduces the invasion of glioma cells via reducing the activity of p38 signalling pathway. PLoS One 2014; 9: e90318.
Demuth T, Reavie LB, Rennert JL, Nakada M, Nakada S, Hoelzinger DB et al. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther 2007; 6: 1212–1222.
Greenberg AK, Basu S, Hu J, Yie TA, Tchou-Wong KM, Rom WN et al. Selective p38 activation in human non-small cell lung cancer. Am J Respir Cell Mol Biol 2002; 26: 558–564.
Besson A, Davy A, Robbins SM, Yong VW . Differential activation of ERKs to focal adhesions by PKC epsilon is required for PMA-induced adhesion and migration of human glioma cells. Oncogene 2001; 20: 7398–7407.
Chuderland D, Seger R . Calcium regulates ERK signalling by modulating its protein-protein interactions. Commun Integr Biol 2008; 1: 4–5.
Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A . A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 2007; 8: 39.
Wang Y, Jiang T . Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett 2012; 331: 139–146.
Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol 1999; 9: 469–479.
Sciuscio D, Diserens AC, van Dommelen K, Martinet D, Jones G, Janzer RC et al. Extent and patterns of MGMT promoter methylation in glioblastoma- and respective glioblastoma-derived spheres. Clin Cancer Res 2011; 17: 255–266.
Bady P, Diserens AC, Castella V, Kalt S, Heinimann K, Hamou MF et al. DNA fingerprinting of glioma cell lines and considerations on similarity measurements. Neuro Oncol 2012; 14: 701–711.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Petrova TV, Nykanen A, Norrmen C, Ivanov KI, Andersson LC, Haglund C et al. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 2008; 13: 407–419.
Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H . Transcriptional activation by tetracyclines in mammalian cells. Science 1995; 268: 1766–1769.
Morgenstern JP, Land H . A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res 1990; 18: 1068.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Cawthorne C, Swindell R, Stratford IJ, Dive C, Welman A . Comparison of doxycycline delivery methods for Tet-inducible gene expression in a subcutaneous xenograft model. J Biomol Tech 2007; 18: 120–123.
Andreeff M, Ruvolo V, Gadgil S, Zeng C, Coombes K, Chen W et al. HOX expression patterns identify a common signature for favorable AML. Leukemia 2008; 22: 2041–2047.
Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND et al. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signalling. Hum Mol Genet 2008; 17: 3105–3117.
O'Connell MP, Fiori JL, Baugher KM, Indig FE, French AD, Camilli TC et al. Wnt5A activates the calpain-mediated cleavage of filamin A. J Invest Dermatol 2009; 129: 1782–1789.
Li X, Zhou Q, Hanus J, Anderson C, Zhang H, Dellinger M et al. Inhibition of multiple pathogenic pathways by histone deacetylase inhibitor SAHA in a corneal alkali-burn injury model. Mol Pharm 2013; 10: 307–318.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M et al. Primer3—new capabilities and interfaces. Nucleic Acids Res 2012; 40: e115.
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80.
Gautier L, Cope L, Bolstad BM, Irizarry RA . affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004; 20: 307–315.
Acknowledgements
The presented work was supported by the Swiss National Science Foundation (31003A_138116 / 1). The results published here are in part based on data generated by The Cancer TCGA Genome Atlas pilot project established by the NCI and NHGRI. Information about TCGA and the investigators and institutions who constitute the TCGA research network can be found at http://cancergenome.nih.gov/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Vassallo, I., Zinn, P., Lai, M. et al. WIF1 re-expression in glioblastoma inhibits migration through attenuation of non-canonical WNT signaling by downregulating the lncRNA MALAT1. Oncogene 35, 12–21 (2016). https://doi.org/10.1038/onc.2015.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.61
This article is cited by
-
Immune-related lncRNAs signature and radiomics signature predict the prognosis and immune microenvironment of glioblastoma multiforme
Journal of Translational Medicine (2024)
-
BET protein inhibition sensitizes glioblastoma cells to temozolomide treatment by attenuating MGMT expression
Cell Death & Disease (2022)
-
Long non-coding RNAs in brain tumors: roles and potential as therapeutic targets
Journal of Hematology & Oncology (2021)
-
Mesenchymal stem cells-derived exosomes for drug delivery
Stem Cell Research & Therapy (2021)
-
Metabolic and transcriptomic profiles of glioblastoma invasion revealed by comparisons between patients and corresponding orthotopic xenografts in mice
Acta Neuropathologica Communications (2021)