Abstract
Background:
The pathophysiology of Hirschsprung’s disease (HSCR) is not fully understood. A significant proportion of patients have persisting bowel symptoms such as constipation, soiling, and enterocolitis despite correctly performed operations. Animal data suggest that stretch-activated 2-pore domain K+ channels play a critical role in the maintenance of intestinal barrier integrity.
Methods:
We investigated TREK-1 protein expression in ganglionic and aganglionic regions of HSCR patients (n = 10) vs. normal control colon (n = 10). Protein distribution was assessed by using immunofluorescence and confocal microscopy. Gene and protein expression were quantified using quantitative real-time polymerase chain reaction, western blot analysis, and densitometry.
Results:
Confocal microscopy of the normal colon revealed strong TREK-1 channel expression in the epithelium. TREK-1-positive cells were decreased in aganglionic and ganglionic bowel compared to controls. TREK-1 gene expression levels were significantly decreased in aganglionic and ganglionic bowel compared to controls (P < 0.05). Western blotting revealed decreased TREK-1 protein expression in aganglionic and ganglionic bowel compared to controls.
Conclusion:
We demonstrate, for the first time, the expression and distribution of TREK-1 channels in the human colon. The decreased TREK-1 expression in the aganglionic and ganglionic bowel observed in HSCR may alter intestinal epithelial barrier function leading to the development of enterocolitis.
Similar content being viewed by others
Main
Hirschsprung’s disease (HSCR), a relatively common cause of intestinal obstruction in the newborn, is characterized by the absence of ganglion cells in the distal bowel and extending proximally for varying distances (1,2,3,4). Hirschsprung’s disease–associated enterocolitis (HAEC) is a life-threatening complication of HSCR, which may manifest either before or after definitive pull-through operation (5). Despite multiple investigative studies, the etiology and pathophysiology of HAEC remain poorly understood. Numerous theories have been put forward to explain its occurrence including intestinal epithelial barrier dysfunction and altered motility patterns (6). It is well recognized that intestinal epithelial barrier dysfunction may allow the passage of macromolecular antigens or noxious substances through the barrier to reach subepithelial regions, coming in contact with immune cells and initiating altered immune responses.
Normal intestinal motility requires the coordinated interaction of the enteric nervous system, smooth muscle cells, interstitial cells of Cajal and recently discovered platelet-derived growth factor-α-positive (PDGFRα+) cells. Together, the concerted interaction of these cells propagates the normal motility in the healthy human colon (7).
TREK-1 (KCNK2) is a mechanosensitive K2P channel that is opened by membrane stretch as well as cell swelling (8). TREK-1 channels are reported to play a critical role in setting membrane potential and regulating responses to stretch and nitrergic stimulation (9). Animal data suggests that stretch-activated potassium channels mediate responses of gastrointestinal smooth muscles to enteric nerve stimulation and stretch. This suggests that these channels may be a mechanical factor in motility patterns of the gut, such as local receptive relaxation. Recently, Huang et al. (10) reported that TREK-1 plays a critical role in the maintenance of intestinal barrier integrity. Disruption of intestinal barrier leads to a number of immune-mediated diseases such inflammatory bowel disease (11). We designed this study to test the hypothesis that the expression of TREK-1 channels is altered in the bowel of patients with HSCR.
Results
Relative mRNA Expression Levels of TREK-1 in HSCR and Controls
The relative mRNA expression level of TREK-1 was significantly decreased (P < 0.05) in the aganglionic and ganglionic HSCR specimens compared to controls ( Figure 1 ).
Relative mRNA expression levels of TREK-1 in Hirschsprung’s disease (HSCR) and controls. Quantitative real-time polymerase chain reaction revealed significantly decreased relative mRNA expression levels of TREK-1 (P < 0.05 by Student’s t-test) in the aganglionic HSCR specimens (n = 10) and ganglionic specimens (n = 10) compared to normal control tissue (n = 10). Results are presented as mean ± SEM. *P < 0.05 by Student’s t-test.
Western Blot
Our Western blot results from three independent experiments showed that the TREK-1 channel protein was expressed in the human colon. The expression was decreased in the aganglionic and ganglionic bowel compared to controls ( Figure 2 ). Densitometry confirmed significantly (P < 0.05) decreased TREK-1 protein expression. Equal loading of electrophoresis gels was confirmed by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) staining of the stripped membranes.
Western blot results of TREK-1 channel protein in the human colon. Western blotting and densitometry quantification of TREK-1 protein expression in Hirschsprung’s disease (HSCR) specimens (n = 10) and controls. Western blot results show that the quantitative decrease of TREK-1 transcripts in HSCR specimens resulted in decreased amounts of TREK-1 protein expression compared to controls. Equal loading of electrophoresis gels was confirmed by GAPDH staining. Values are given as mean ± SEM. *P < 0.05 by Student’s t-test.
Immunofluorescence Staining and Confocal Microscopy
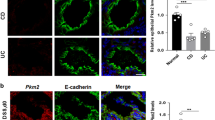
TREK-1 protein showed strong colocalization with E-Cadherin ( Figure 3 ). Confocal microscopy validated the western blot findings showing a decreased TREK-1 protein expression in aganglionic and ganglionic HSCR specimens vs. controls.
Immunofluorescence staining and confocal microscopy. TREK-1 channel expression in (a) aganglionic, (b) ganglionic, and (c) normal colonic tissue (green) and E-Cadherin (red). E-Cadherin was used to identify colonic epithelium and to show coexpression with TREK-1 protein (original magnification ×63; scale bar 25 µm).
Discussion
To our knowledge, this is the first report describing TREK-1 expression in the human colon and HSCR. Confocal microscopy revealed that TREK-1-positive cells were markedly decreased in the aganglionic and ganglionic bowel of HSCR patients compared to controls. Similarly, TREK-1 gene and protein expression was significantly decreased in the aganglionic and ganglionic segments of HSCR patients compared to controls.
The TREK-1 subfamily (TREK-1, TREK-2, and TRAAK) belongs to the two-pore domain K+ (K2P or KCNK) channels. These channels have four transmembrane domains and two pore domains. Two subunits of these channels form functional channels (12). Although its original function was considered to be for K+ ion transportation, recent reports have revealed that TREK-1 is implicated in the maintenance of epithelial and endothelial barrier integrity (10,13). Huang et al. (10) reported that TREK-1 deficiency induced barrier dysfunction in a human colon epithelial cell line and their data strongly suggested that TREK-1 is required for the maintenance of intestinal epithelial barrier integrity.
HAEC is a serious life-threatening condition with a reported incidence of 20 to 35%. Post-pull-through HAEC has been reported in 5–44% of HSCR patients (5,6,14,15). Since HAEC can occur following a definitive pull-through operation, it is suggested that the proximal ganglionic bowel of HSCR patients is dysfunctional. Our results of this study support this concept. All HSCR specimens expressed the transcript for the TREK-1 channel, followed by the translation of the full protein; thus, showing that TREK-1 gene and protein expression are significantly reduced in the aganglionic and ganglionic specimens of HSCR compared to normal controls.
Despite recent advances in understanding HAEC, the pathogenesis of HAEC is poorly understood.
Alterations in the functional anatomic integrity of the intestinal barrier have been suggested as one of the critcal factors in the pathophysiological processes of HAEC (16). The intestinal barrier function in the gut begins within lumen of the bowel as the first line, followed by the mucus layer. The next line of defense of the intestinal barrier is the epithelial layer (5). Under normal conditions, enterocyte tight junction (TJ) proteins allow the gut epithelium to function as a barrier yet absorb substances in a paracellular manner (17). TJ proteins are macromolecular complexes including membrane proteins such as claudins, scaffolding proteins as well as elements of the cytoskeleton (18). Disturbances of epithelial barrier function have been reported in several conditions of intestinal inflammation (11).
There is a dynamic interaction between TREK-1 and the actin cytoskeleton (19). The expression of TREK-1 has profound effect on the actin network architecture and their function in mechano-sensation is well described (20,21). It has been reported that TREK-1-deficient cells contain less amounts of F-actin (19). Since TREK-1 is closely related to actin cytoskeleton, TREK-1 deficiency may alter the TJ-associated proteins that seal the TJs, thus allowing increased permeability to noxious substances. TJ are features of differentiated epithelial cells that regulate the integrity and permeability of tissue barriers. Structure and remodeling of epithelial junctions depend on their association with the underlying actomyosin cytoskeleton (18). It is described that even single changes in associated scaffolding proteins can lead to junctional disassembly and dramatic disorganization of the perijunctional actinomycin, and, thus influencing epithelial barrier function (22).
A recent study reported that TREK-1 deficiency in TREK-1 KO mice resulted in increased lung damage (23). The authors proposed that the deficiency could have an impact on cytokine secretion and epithelial barrier function, and that the dysregulation of these proteins can contribute to the pathogenesis of epithelial injury. Furthermore, they documented an increased secretion of cytokines such as IL-6, IL-1β, and TNF-α (23). It is well known that the above cytokines contribute to the development of intestinal barrier dysfunction (24) by mechanisms such as increasing the paracellular permeability of the intestinal mucosa (25). The proinflammatory environment associated with the secretion of these mediators promotes neutrophil-, macrophage-, T-cell, and epithelium-mediated injury resulting in loss of barrier function. Furthermore, Jiang et al. (13) reported, that nasal epithelia express TREK-1; and that the suppression of TREK-1 with IL-4 via an upregulated expression of histone deacetylase leads to nasal epithelial dysfunction. All the above studies suggest a major role of TREK-1 in the maintenance of the epithelial barrier function in different tissues. Four of the 10 patients developed enterocolitis, two having Trisomy 21, one had total colonic aganglionosis, and the fourth patient had rectosigmoid HSCR. However, the TREK-1expression in the aganglionic and ganglionic bowel was not different from HSCR patients with enterocolitis from those who did not have enterocolitis. This finding suggests that the role of TREK-1 in modulating the epithelial barrier function leading to the development of HAEC may be dependent on additional factors such as altered microbiome, impaired innate immune, and impaired adaptive immunity. Further studies are needed to elucidate the underlying mechanisms.
There is increasing evidence that intestinal barrier dysfunction is a critical factor in the pathogenesis of HAEC (26,27,28). Changes in the the colonic epithelium have been postulated as a causative factor in the development of HAEC through pertubations in structure and function of the epithelial lining (29). Recently, we showed that ATP-sensitive K+-channel (K(ATP)) subunits are which colocalized with TJ-proteins were reduced in Hirschsprung’s specimens (30). These findings implicate an intrinsic deficiency of K+ channels, in the development of HAEC. Additional investigations in this exciting area using recent advances in molecular technology to assess intestinal barrier function should provide new insights into the mechanims underlying HAEC.
Methods
Tissue Samples
This study was approved by the Ethics Medical Research Committee, Our Lady’s Children’s Hospital Crumlin (Ref GEN.292/12) and tissue samples were obtained with informed parental consent. HSCR specimens from 10 patients (7 male, 3 female, 3–14 mo, Table 1 ) who underwent pull-through surgery were studied. These specimens were divided into aganglionic and ganglionic samples. Ganglionic samples were taken from the most proximal margin of the pull-through specimen while aganglionic samples were taken from the most distal margin of the pull-through specimens. Normal control samples included 10 specimens from patients who underwent sigmoid colostomy closure following anorectoplasty for imperforate anus (6 male, 4 female, 8–19 mo). Tissue specimens were either snap-frozen in liquid nitrogen and stored at −80 °C for protein extraction or embedded in OCT mounting compound (VWR International, Leuven, Belgium) for immunofluorescence and stored at −80 °C until use.
RNA Isolation From HSCR Specimens
Isolate II RNA Mini Kit was used to isolate total RNA from aganglionic and ganglionic HSCR as well as controls (n = 10 for each group) according to the manufacturer’s protocol. Spectrophotometrical quantification of total RNA was performed using a NanoDrop ND-1000 UV-Vis spectrophotometer (Thermo Scientific Fisher, Wilmington, DE). The RNA solution was stored at −80 °C until further use.
cDNA Synthesis and Quantitative PCR
Reverse transcription of total RNA was carried out at 25 °C for 10 min, at 37 °C for 120 min and at 85 °C for 5 min using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, West Sussex, UK) according to the manufacturer’s instruction. The resulting cDNA was used for quantitative real-time polymerase chain reaction (qRT-PCR) using a LightCycler 480 SYBR Green I Master (Roche Diagnostics, Mannheim, Germany) in a total reaction mix of 25 µl per well. The following gene-specific primers were used: Human TREK-1 sense primer 5′ CAATTCGACGGAGCTGGATG and Human TREK-1 anti-sense primer 5′ CTTCTGTGCGTGGTGAGATG (Eurofins). After 5 min of initial denaturation at 95 °C, 55 cycles of amplification for each primer were carried out. Each cycle included denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s, and elongation at 72 °C for 10 s. Relative mRNA levels of gene expression were determined using a LightCycler 480 System (Roche Diagnostics). The relative changes in gene expression levels of TREK-1 were normalized against the level of GAPDH gene expression in each sample (ΔΔCT-method). Experiments were carried out in triplicate for each sample and primer.
Protein Extraction and Western Blot
Specimens of HSCR colon and control colon were homogenized in RIPA buffer (Radio-Immunoprecipitation Assay, Sigma-Aldrich, Wicklow, Ireland) containing 1% protease inhibitor cocktail (Sigma-Aldrich Ireland). Protein concentrations were determined using a Bradford assay (Sigma-Aldrich Ireland). A total volume of 20 µl Laemmli Sample Buffer (Sigma-Aldrich, Ireland) containing 10 µg protein was loaded in the 10% SDS-PAGE gel (NuPAGE Novex Bis-Tris gels, Invitrogen, Carlsbad, CA) for electrophoretic separation. The electrophoresis was performed in 2-(N-morpholino)ethanesulfonic acid (MES) sodium dodecyl sulfate (SDS) running buffer (Invitrogen). Proteins were then transferred to 0.45 µm nitrocellulose membrane (Millipore Corporation, Billerica, MA) by western blotting. Following western blotting, the membranes were blocked with 3% skimmed milk for 60 min before antibody detection. The primary antibody; rabbit anti-TREK-1 (Abcam, Cambridge, UK) dilution 1:1,000, was used, and incubation was performed overnight at 4 °C. Following washing (four times in PBS—0.05% Tween), the membranes were incubated with goat anti-rabbit IgG HRP-linked secondary antibody (dilution 1:10,000, Abcam) followed by washing (four times in phosphate-buffered saline (PBS)—0.05% Tween). Detection was performed with the ECL Plus chemiluminescence kit (Thermo, Fisher Scientific, Dublin, Ireland). We used GAPDH (mouse anti-GAPDH, dilution 1:1,000, Abcam) as an additional loading control.
Immunofluorescence Staining and Confocal Microscopy
Frozen blocks of HSCR colon and normal control samples were sectioned transversely at a thickness of 10 µm, mounted on Superfrost Plus slides (VWR International, Leuven, Belgium) and fixed with buffered 10% formalin for 10 min. Sections underwent cell membrane permeabilization with 1% TritonX-100 for 25 min at room temperature. After blocking with 5% bovine serum albumin (BSA) for 30 min to avoid nonspecific absorption, sections were incubated with primary antibodies: rabbit anti-TREK-1, (1:100, BSA 5%), (Santa Cruz, Heidelberg, Germany), and mouse anti-E-Cadherin (1:100, 5% BSA), (Santa Cruz) overnight at 4 °C. Sections were then washed in PBS—0.05% Tween and incubated with corresponding secondary antibodies (anti-rabbit Alexa Fluor 488, dilution 1:1,000 and anti-mouse Alexa Fluor594, dilution 1:1,000, Abcam) for 1 h at room temperature. After washing, sections were counterstained with 4′,6-diamidino-2-phenylindole antibody, dilution 1:1,000 (Roche Diagnostics GmbH, Mannheim, Germany) for 15 min, washed, mounted and coverslipped with Fluorescent Mounting Medium (DAKO, Cambridgeshire, UK). All sections were independently evaluated by two investigators with a LSM 700 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Statistical Analysis
All numerical data are presented as mean ± SEM. Student’s t-test was used for evaluation of differences between the aganglionic, ganglionic, and normal controls. The confidence interval was set at 95%.
Disclosure
We have no conflicts of interest to disclose. The authors have no financial relationships relevant to this article to disclose.
References
Puri P, Ohshiro K, Wester T. Hirschsprung’s disease: a search for etiology. Semin Pediatr Surg 1998;7:140–7.
Puri P, Montedonico S. Hirschsprung’s disease: clinical features. In: Hirschsprung’s Disease and Allied Disorders. Springer, Berlin-Heidelberg; 2008:107–113.
Peña A, Bischoff A, Barnes L. Surgical Treatment of Colorectal Problems in Children. Cham: Springer International Publishing; 2015
Holschneider AM, Puri P. Hirschsprung’s Disease and Allied Disorders. Springer; 2008
Gosain A, Brinkman AS. Hirschsprung’s associated enterocolitis. Curr Opin Pediatr 2015;27:364–9.
Gosain A, Barlow-Anacker AJ, Erickson CS, et al. Impaired cellular immunity in the murine neural crest conditional deletion of endothelin receptor-B model of Hirschsprung’s disease. PLoS One 2015;10:e0128822.
Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil 2014;20:294–317.
Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honoré E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J 2005;24:44–53.
Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J Physiol 2006;570(Pt 1):37–43.
Huang H, Liu JQ, Yu Y et al. Regulation of TWIK-related potassium channel-1 (Trek1) restitutes intestinal epithelial barrier function. Cell Mol Immunol 2016;13:110–8.
Snoek SA, Verstege MI, Boeckxstaens GE, van den Wijngaard RM, de Jonge WJ. The enteric nervous system as a regulator of intestinal epithelial barrier function in health and disease. Expert Rev Gastroenterol Hepatol 2010;4:637–51.
Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J Physiol 2006;570(Pt 1):37–43.
Jiang J, Liu JQ, Li J, et al. Trek1 contributes to maintaining nasal epithelial barrier integrity. Sci Rep 2015;5:9191.
Demehri FR, Halaweish IF, Coran AG, Teitelbaum DH. Hirschsprung-associated enterocolitis: pathogenesis, treatment and prevention. Pediatr Surg Int 2013;29:873–81.
Neuvonen MI, Kyrklund K, Lindahl HG, Koivusalo AI, Rintala RJ, Pakarinen MP. A population-based, complete follow-up of 146 consecutive patients after transanal mucosectomy for Hirschsprung disease. J Pediatr Surg 2015;50:1653–8.
Austin KM. The pathogenesis of Hirschsprung’s disease-associated enterocolitis. Semin Pediatr Surg 2012;21:319–27.
Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733.
D’Atri F, Citi S. Molecular complexity of vertebrate tight junctions (Review). Mol Membr Biol 2002;19:103–12.
Roan E, Waters CM, Teng B, Ghosh M, Schwingshackl A. The 2-pore domain potassium channel TREK-1 regulates stretch-induced detachment of alveolar epithelial cells. PLoS One 2014;9:e89429.
Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem 1999;274:26691–6.
Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J Biol Chem 2000;275:10128–33.
Wang D, Chadha GK, Feygin A, Ivanov AI. F-actin binding protein, anillin, regulates integrity of intercellular junctions in human epithelial cells. Cell Mol Life Sci 2015;72:3185–200.
Brune K, Frank J, Schwingshackl A, Finigan J, Sidhaye VK. Pulmonary epithelial barrier function: some new players and mechanisms. Am J Physiol Lung Cell Mol Physiol 2015;308:L731–45.
Gyires K, Tóth ÉV, Zádori SZ. Gut inflammation: current update on pathophysiology, molecular mechanism and pharmacological treatment modalities. Curr Pharm Des 2014;20:1063–81.
Alnabhani Z, Montcuquet N, Biaggini K, et al. Pseudomonas fluorescens alters the intestinal barrier function by modulating IL-1β expression through hematopoietic NOD2 signaling. Inflamm Bowel Dis 2015;21:543–55.
Pierre JF, Barlow-Anacker AJ, Erickson CS, et al. Intestinal dysbiosis and bacterial enteroinvasion in a murine model of Hirschsprung’s disease. J Pediatr Surg 2014;49:1242–51.
Thiagarajah JR, Yildiz H, Carlson T, et al. Altered goblet cell differentiation and surface mucus properties in Hirschsprung disease. PLoS One 2014;9:e99944.
Ward NL, Pieretti A, Dowd SE, Cox SB, Goldstein AM. Intestinal aganglionosis is associated with early and sustained disruption of the colonic microbiome. Neurogastroenterol Motil 2012;24:874–e400.
Gosain A. Established and emerging concepts in Hirschsprung’s-associated enterocolitis. Pediatr Surg Int 2016;32:313–20.
Tomuschat C, O’Donnell AM, Coyle D, Dreher N, Kelly D, Puri P. Altered expression of ATP-sensitive K(+) channels in Hirschsprung’s disease. J Pediatr Surg 2016;51:948–52.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomuschat, C., O’Donnell, A., Coyle, D. et al. Altered expression of a two-pore domain (K2P) mechano-gated potassium channel TREK-1 in Hirschsprung’s disease. Pediatr Res 80, 729–733 (2016). https://doi.org/10.1038/pr.2016.140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.140
This article is cited by
-
A successful centre for translational paediatric surgical research
Pediatric Surgery International (2022)
-
Translational research in Hirschprung’s disease at the National Children’s Research Centre in Dublin
Pediatric Surgery International (2022)
-
A pilot study characterizing longitudinal changes in fecal microbiota of patients with Hirschsprung-associated enterocolitis
Pediatric Surgery International (2022)
-
Prevalence of Hirschsprung-associated enterocolitis in patients with Hirschsprung disease
Pediatric Surgery International (2022)
-
K2P2.1 (TREK-1) potassium channel activation protects against hyperoxia-induced lung injury
Scientific Reports (2020)