Abstract
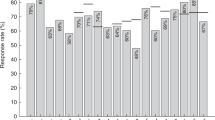
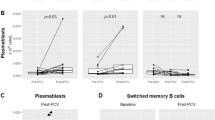
Pneumococcal (PC) vaccination is recommended for patients with chronic lymphocytic leukemia (CLL). However, response to vaccines has been investigated in a small series of CLL patients. We analyzed the antibody response and outcomes of 112 CLL patients who received the 13-valent pneumococcal conjugate vaccine (PCV13). An immune response was defined by a twofold increase in the PC-IgG levels assessed by ELISA. The median age of patients was 68 years, 23.2% showed IgG levels ≤ 400 mg/L, 6.3% progressive disease, 52% unmutated IGHV. Twenty-two (19.6%) patients were treatment-naïve and 90 (80.4%) previously treated (40.2% front-line chemoimmunotherapy; ibrutinib first/advanced-line, 9.8%/21.4%; idelalisib advanced-line, 8.9%). Nine (8%) patients developed an immune response, eight treatment-naive, and one on front-line ibrutinib. No responses were observed in patients previously treated with chemoimmunotherapy. Age ≥ 60 years (p = 0.007), IgG levels < 400 mg/L (p < 0.0001), prior treatment (p < 0.0001), and signs of disease progression (p = 0.04) were associated with a lower response rate. Pneumonia-free survival was significantly shorter in patients with clinical signs of progressive disease (HR, 8.39), prior pneumonia (HR, 7.03), and TP53 disruption (HR, 2.91). In conclusion, our results suggest that vaccination should be offered at diagnosis to CLL patients with early stage and stable disease who have better resources for an effective immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
National Cancer Institute. Surveillance, Epidemiology and End Results Program; 2007–2013. https://seer.cancer.gov/statfacts/html/clyl.html.
Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Prim. 2017;3:17008.
Ravandi F, O’Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother. 2006;55:197–209.
Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–81.
Rozman C, Montserrat E, Viñolas N. Serum immunoglobulins in B-chronic lymphocytic leukemia. Nat Hist Progn Signif Cancer. 1988;15;61:279–83.
Shvidel L, Tadmor T, Braester A, Bairey O, Rahimi-Levene N, Herishanu Y, et al. Serum immunoglobulin levels at diagnosis have no prognostic significance in stage A chronic lymphocytic leukemia: a study of 1113 cases from the Israeli CLL Study Group. Eur J Haematol. 2014;93:29–33.
Parikh SA, Leis JF, Chaffee KG, Call TG, Hanson CA, Ding W, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: natural history, clinical correlates, and outcomes. Cancer. 2015;1;121:2883–91.
Reda G, Cassin R, Gentile M, Mauro FR, Giannarelli D, Fattizzo B, et al. IgA hypogammaglobulinemia predicts outcome in chronic lymphocytic leukemia. Leukemia. 2019;33:1519–22.
Mauro FR, Morabito F, Vincelli ID, Petrucci L, Campanelli M, Salaroli A, et al. Clinical relevance of hypogammaglobulinemia, clinical and biologic variables on the infection risk and outcome of patients with stage A chronic lymphocytic leukemia. Leuk Res. 2017;57:65–71.
Morrison VA. Infections in patients with leukemia and lymphoma. Cancer Treat Res. 2014;161:319–49.
Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:240–9.
Pleyer C, Wiestner A, Sun C. Immunological changes with kinase inhibitor therapy for chronic lymphocytic leukemia. Leuk Lymphoma. 2018;59:2792–2800.
Williams AM, Baran AM, Meacham PJ, Feldman MM, Valencia HE, Newsom-Stewart C, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma. 2018;59:625–32.
Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis. 2018;16;67:687–92.
Teh BW, Tam CS, Handunnetti S, Worth LJ, Slavin MA. Infections in patients with chronic lymphocytic leukaemia: Mitigating risk in the era of targeted therapies. Blood Rev. 2018;32:499–507.
Rozenbaum MH, Pechlivanoglou P, Van der Werf TS, Lo-Ten-Foe JR, Postma MJ, Hak E. The role of Streptococcus pneumoniae in community-acquired pneumonia among adults in Europe: a meta-analysis. Eur J Clin Microbiol Infect Dis. 2013;32:305–16.
Curcio D, Cane A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis. 2015;37:30–35.
Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;12;61:816–9.
Kobayashi M, Bennett NM, Gierke R, Gierke R, Almendares O, Moore MR, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;4;64:944–7.
Pedrazzoli P, Piralla A, Valentino F, Cinieri S, Baldanti F. Update of the recommendations of the Italian Society of Medical Oncology on vaccination for seasonal influenza and pneumococcal infection in patients with cancer: Focus on prevention of pneumonia. Eur J Cancer Care. 2018;27:e12817.
Backhaus E, Berg S, Andersson R, Ockborn G, Malmström P, Dahl M, et al. Epidemiology of invasive pneumococcal infections: manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect Dis. 2016;3:367–79.
Mikulska M, Cesaro S, de Lavallade H, Di Blasi R, Einarsdottir S, Gallo G, et al. European Conference on Infections in Leukaemia group. Vaccination of patients with haematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e188–e199.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;21;131:2745–60.
Wierda WG, Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Andreadis CB, et al. NCCN Guidelines Insights: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 1.2017. J Natl Compr Canc Netw. 2017;15:293–311.
Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M. et al. ESMO Guidelines Committee. appendix 6: chronic lymphocytic leukaemia: eUpdate published online September 2016. Ann Oncol. 2016;27v143–v144.
Schuh AH, Parry-Jones N, Appleby N, Bloor A, Dearden CE, Fegan C, et al. Guideline for the treatment of chronic lymphocytic leukaemia: a British Society for Haematology Guideline. Br J Haematol. 2018;182:344–59.
Sinisalo M, Aittoniemi J, Oivanen P, Käyhty H, Ölander R, Vilpo J. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia. Br J Haematol. 2001;114:107–10.
Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19:1671–7.
Pasiarski M, Rolinski J, Grywalska E, Stelmach-Goldys A, Korona-Glowniak I, Gozdz S, et al. Antibody and plasmablast response to 13-valent pneumococcal conjugate vaccine in chronic lymphocytic leukemia patients-preliminary report. PLoS ONE. 2014;9:e114966.
Svensson T, Kättström M, Hammarlund Y, Roth D, Andersson PO, Svensson M, et al. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the swedish CLL group. Vaccine. 2018;36:3701–7.
Andrick B, Alwhaibi A, DeRemer DL, Quershi S, Khan R, Bryan LJ, et al. Lack of adequate pneumococcal vaccination response in chronic lymphocytic leukaemia patients receiving ibrutinib. Br J Haematol. 2018;182:712–4.
Sun C, Gao J, Couzens L, Tian X, Farooqui MZ, Eichelberger MC, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with Ibrutinib. JAMA Oncol. 2016;1;2:1656–7.
Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017;102:e397–e399.
Kapetanovic MC, Nagel J, Nordström I, Saxne T, Geborek P, Rudin A. Methotrexate reduces vaccine-specific immunoglobulin levels but not numbers of circulating antibody-producing B cells in rheumatoid arthritis after vaccination with a conjugate pneumococcal vaccine. Vaccine. 2017;35:903–8.
Winthrop KL, Bingham CO 3rd, Komocsar WJ, Bradley J, Issa M, Klar R, et al. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther. 2019;18;21:102.
Parker AR, Park MA, Harding S, Abraham RS. The total IgM, IgA and IgG antibody responses to pneumococcal polysaccharide vaccination (Pneumovax®23) in a healthy adult population and patients diagnosed with primary immunodeficiencies. Vaccine. 2019;28;37:1350–5.
Moreira J, Rabe KG, Cerhan JR, Kay NE, Wilson JW, Call TG, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia. 2013;27:136–41.
Hensel M, Kornacker M, Yammeni S, Egerer G, Ho AD. Disease activity and pretreatment, rather than hypogammaglobulinaemia, are major risk factors for infectious complications in patients with chronic lymphocytic leukaemia. Br J Haematol. 2003;122:600–6.
Visentin A, Imbergamo S, Gurrieri C, Frezzato F, Trimarco V, Martini V, et al. Major infections, secondary cancers and autoimmune diseases occur in different clinical subsets of chronic lymphocytic leukaemia patients. Eur J Cancer. 2017;72:103–11.
Smolej L. Efficacy of pneumococcal vaccination in chronic lymphocytic leukemia: should we rey on surrogate markers? Vaccine. 2008;10;26:1407.
O’Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;26;131:1910–9.
Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SE, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126:2213–9.
Coutre SE, Byrd JC, Hillmen P, Barrientos JC, Barr PM, Devereux S, et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;25;3:1799–807.
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;29;123:3390–7.
EM Agency. EMA recommends new safety measures for Zydelig. European Medicines Agency; 2016. https://www.ema.europa.eu/en/documents/referral/zydelig-article-20-procedure-chmp-confirms-recommendations-use-zydelig_en-0.pdf.
Maschmeyer G, De Greef J, Mellinghoff SC, Nosari A, Thiebaut-Bertrand A, Bergeron A, et al. European Conference on Infections in Leukemia (ECIL). Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia 2019;33:844–62.
Reinwald M, Silva JT, Mueller NJ, Fortún J, Garzoni C, de Fijter JW, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(Suppl 2):S53–S70.
Acknowledgements
The authors would like to thank the ‘Sapienza’ University of Rome and the Fellowship Program of Gilead, who provided financial support for the management of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
This study was carried out according to the local requirements for safety reporting to Health Authorities and or Independent Ethics Committees (IECs)/Institutional Review Boards (IRBs).
Informed consent
Patients were asked to provide informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mauro, F.R., Giannarelli, D., Galluzzo, C.M. et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia 35, 737–746 (2021). https://doi.org/10.1038/s41375-020-0884-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0884-z
This article is cited by
-
Impfungen in der Hämatologie und Onkologie
best practice onkologie (2023)
-
Effectiveness, immunogenicity, and safety of COVID-19 vaccines for individuals with hematological malignancies: a systematic review
Blood Cancer Journal (2022)
-
Managing the Risk of Infection in Chronic Lymphocytic Leukemia in the Era of New Therapies
Current Oncology Reports (2022)
-
Cellular and humoral immune response to SARS-CoV-2 mRNA vaccines in patients treated with either Ibrutinib or Rituximab
Clinical and Experimental Medicine (2022)
-
Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia
Blood Cancer Journal (2021)