Abstract
Monitoring of cerebral oxygenation (rScO2) with near-infrared spectroscopy (NIRS) is a feasible noninvasive bedside technique in the NICU. This review discusses the possible neuroprotective role of “pattern recognition” of NIRS-derived rScO2 in preterm neonates with regard to the prevention of severe intraventricular hemorrhage and hypoxia/hyperoxia-related white matter injury. This neuroprotective role of rScO2 monitoring is discussed as a modality to aid in the early detection of cerebral oxygenation conditions predisposing to these complications. Practical guidelines are provided concerning management of abnormal rScO2 patterns as well as a brief discussion concerning the need for international consensus and the legal aspects associated with the introduction of a new NICU bedside monitoring strategy.
Similar content being viewed by others
Introduction
Near-infrared spectroscopy (NIRS) offers the ability to noninvasively monitor alterations in oxygenation, oxygen extraction, and perfusion of the immature brain.1,2,3 A recent study showed that NIRS-derived regional cerebral saturation (rScO2; %), which is a mixture of venous (∼75%), capillary (∼5%), and arterial saturation (∼20%), correlates well with magnetic resonance imaging (MRI)-determined cerebral oxygenation.4 Further, combining NIRS-monitored rScO2 with simultaneous mean arterial pressure can be used as a clinical tool to assess and trend cerebral autoregulation in both premature and term neonates.5,6 A recent multicenter interventional study, the SafeboosC Study, demonstrated that cerebral oxygenation was quite stable and mostly within the expected reference ranges (55–85%) in 166 extremely preterm neonates during the first 72 h of postnatal life when rScO2 was monitored.7,8 Moreover, decreased hypoxia burden was associated with a lower incidence of severe intraventricular hemorrhage in the rScO2-monitored cohort.9 This result was consistent with previous data demonstrating an association between both cerebral hyperoxia and hypoxia with adverse long-term neurodevelopmental outcome.10
Nonetheless, though commonly encountered in the research setting, the clinical application of cerebral oxygenation monitoring in the neonatal intensive care unit (NICU) remains highly limited. Indeed, NIRS-based cerebral oxygenation monitoring remains at best a “trend monitoring” technique, based on variable precision and data averaging techniques across a range of studies.11,12,13,14 Moreover, other studies have shown differences in rScO2 based on utilizing different NIRS devices and sensors, most likely due to variable algorithms being employed.12,15 Finally, uncertainty beneath which thresholds tissue oxygen utilization becomes delivery-dependent has limited its widespread adoption.12 However, from a “trend monitoring” perspective, NIRS-derived cerebral oxygenation may provide valuable bedside information with implications for clinical management. If merely used for “pattern recognition”, such monitoring may be considered a potential early warning sign prompting further evaluation, especially if the values of rScO2 remain outside the published reference ranges.8,15
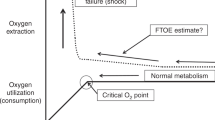
In this review, we will focus on the potential utility of continuous NIRS-based cerebral tissue oxygenation monitoring as a component of a neuroprotective strategy in premature neonates (Fig. 1). A more detailed explanation of the NIRS monitoring technique, including its validation and general reference ranges, is beyond the focus of this review, though it is discussed in numerous other articles.1,2,8,11,12
Cerebral oxygenation monitoring and the preterm neonate
Periventricular–intraventricular hemorrhage (PIVH) and diffuse white matter injury (WMI) are important contributors to adverse neurodevelopmental outcomes among preterm neonates.16,17,18 The neuroprotective role of NIRS-based cerebral oxygenation monitoring via general “pattern recognition” techniques are detailed below. In the present review, we discuss strategies to aid in the prevention of severe PIVH, followed by an overview of techniques to potentially reduce the burden of cerebral hypoxia/hyperoxia with the goal of reducing WMI in the preterm population.
Prevention of severe intraventricular hemorrhage
The pathogenesis of PIVH, although multifactorial,18,19 is firmly linked to immaturity of the preterm infant and more specifically to vascular immaturity within the germinal matrix, the usual origin of PIVH.20 Immaturity of several organ systems, in particular the lungs, with or without inflammation, results in neonatal respiratory distress syndrome (RDS), which facilitates important clinical conditions predisposing for extension of PIVH. The most important etiologic factors for PIVH in this respect are hypercapnia, lack of cerebral autoregulation, and apparent hypotension often resulting in anti-hypotensive treatment. Hypercapnia-induced cerebral hyperperfusion,18,21 a pressure-passive perfusion of the brain,22 and hypotension-induced use of inotropic agents21 are all related to the occurrence and extension of PIVH. Monitoring rScO2 patterns can aid clinicians in identifying and potentially correcting these conditions during earlier stages.
Hypercapnia-induced cerebral hyperperfusion
Ongoing assessment of rScO2 patterns has been shown to provide information on hypercapnia-induced cerebral hyperperfusion. Awareness of abnormal rScO2 trending during hypercapnia, demonstrated in Fig. 2a, may result in earlier detection and correction of severe hypercapnia in mechanically ventilated infants with consequent normalization of brain perfusion and oxygenation. Since acute increases in PaCO2, even within normal limits, can result in increased cerebral blood flow,23 employing tight PaCO2 limits during the first 72 postnatal hours could conceivably improve the stability of cerebral perfusion as indicated by a stable rScO2 within normal limits.
a The patterns of rScO2 (in red box) and end-tidal (et) CO2 (purple: as a surrogate for arterial PaCO2) both decreasing from supranormal values, indicating hyperoxygenation/overperfusion (rScO2: 91%) and hypercapnia (etCO2: 58 mmHg), respectively, to normal values (72% and 41 mmHg, respectively; orange arrows) after ventilator adjustment. Note the normal arterial pressure (MABP) and that a normal arterial oxygen saturation (SaO2) is not indicative of normal cerebral oxygenation. Adapted from Dix et al.2 b The red box includes the pattern of cerebral oxygenation (blue) as represented by rScO2, which passively follows mean arterial blood pressure (MABP; red) in a girl born at 26 0/7 weeks on postnatal day 1, strongly suggesting lack of cerebral autoregulation.
Pressure-passive brain perfusion
Impaired cerebral autoregulation can be recognized in rScO2 patterns which passively follow the mean arterial blood pressure,5,6 as depicted in Fig. 2b. Consequently, this monitoring technique may aid in identifying neonates particularly sensitive to handling who may benefit from sedation to minimize swings in cerebral perfusion in the context of impaired cerebral autoregulation. In addition, among hypotensive premature neonates, examining the relationship between blood pressure and rScO2 may allow clinicians to better select those patients who would most benefit from inotropic support rather than relying on mean arterial blood pressure alone (see also below).
Hypotension-induced use of inotropic agents
With regard to blood pressure management, a prior study by the Utrecht group suggested that neonatal hypotension, defined as a mean arterial blood pressure lower than the gestational age at birth, is unreliable and possibly associated with overtreatment.24 Moreover, Alderliesten et al.21 reported that inotropes, such as dopamine, can induce cerebral hyperperfusion and loss of cerebral autoregulation among premature neonates. Figure 2c represents a typical example. They further reported that elevated rScO2 values, with resultant cerebral hyperperfusion and loss of cerebral autoregulation, preceded the occurrence and in particular the extension of already-existing PIVHs.21 The same group reported that hypotension alone, as defined using the above gestational age criteria, was neither related to low rScO2 values nor to impaired neurodevelopmental outcome at 2 years of age.25 Currently, the TOHOP study, a single-center, randomized study in the Utrecht NICU is assessing “permissive” hypotension in the study group based on rScO2 values remaining within the normal limits (55–85% as investigated in a prior prospective study8 in addition to normal blood gases, urine output, and capillary refill).
Taken together, these observations may result in improved personalization of critical care such that more appropriate, and potentially neuroprotective, strategies may be employed where needed. Cerebral oxygenation monitoring may set the stage for more timely and patient-specific management, with potential reduction in PIVH and ultimately improved neurodevelopmental outcomes.
Table 1 summarizes the most important conditions related to elevated rScO2 values.
Prevention of WMI
WMI occurs in up to 50% of extremely and very preterm neonates according to several recent MRI studies,26,27,28 with substantial implications for neurodevelopmental outcome (25–50%) and cerebral paresis (5–10%).29 Although antenatal WMI has been well described due to maternal/fetal infection or fetal hypoxia18,30 and is beyond the focus of this review, postnatal cerebral hypoxia–ischemia (HI) is also a potentially important etiologic factor for WMI. The conditions associated with cerebral hypoperfusion and subsequent HI include hypocapnia;31,32 hemodynamically significant patent ductus arteriosus (hsPDA);33,34 severe anemia and/or blood loss;35,36,37 hypoglycemia;38 and progressive posthemorrhagic ventricular dilatation (PHVD).35,39 Monitoring the rScO2 patterns can potentially aid clinicians in identifying and correcting these conditions at an early stage, with the goal of preventing or attenuating HI and ultimately improving outcomes.
Hypocapnia
Conversely to hypercapnia-induced cerebral hyperperfusion, acute hypocapnia can cause sudden decreases in cerebral perfusion.23 Special attention to this phenomenon is particularly important in mechanically ventilated infants should overventilation occur. Of note, PaCO2 values below 30 mmHg have been associated with significant effects on cerebral perfusion40 (Fig. 3a). In a cerebral monitoring paradigm, decreasing rScO2 to abnormally low values in a ventilated neonate could serve as an early warning sign for overventilation, prompting assessment of PaCO2 as a potential etiology.
a The patterns of arterial saturation (SaO2;orange), and rScO2 (blue) and mean arterial blood pressure (MABP; red) of an extremely preterm infant on postnatal day 1. The initial rScO2 values were very low (red box). These low values seemed to be associated with PaCO2 values below 30 mmHg (brown squares; starting at 24 mmHg, see also brown arrows). SaO2 and MABPs values were always normal. When PaCO2 values increased above values of 30 mmHg the rScO2 increased and eventually normalized. b The patterns of rScO2 (blue) and mean arterial blood pressure (MABP; red) of a very preterm girl, starting on postnatal day 1, was especially marked by a steep decrease in cerebral oxygenation (rScO2; red box) to very low values (<40%). Echocardiographic investigation early on postnatal day 2 revealed a hemodynamically significant ductus arteriosus. Subsequent ductal closure with indomethacin (2 courses) was followed by normalization of cerebral oxygenation. c The patterns of heart rate (HR), arterial saturation (SaO2) and rScO2 (red box) in a preterm neonate.with severe anemia. The rather low rScO2 recovered following packed red blood cell transfusion (courtesy Prof. Gunnar Naulaers, UZ Leuven).
Hemodynamically significant PDA
Although management of hsPDA remains a controversial issue,41,42 cerebral oxygenation can be impaired by an hsPDA, with potentially severe ranging rScO2 values below 40–45% (Fig. 3b).34 Furthermore, prolonged duration (>30–60 min) of cerebral hypoxia at these low values has been associated with cerebral injury and/or impaired cerebral metabolism in several experimental and clinical studies.43,44,45,46 Previous studies in extremely preterm neonates have demonstrated a negative impact of hsPDA on cerebellar growth.47,48 In a large cohort of preterm infants with hsPDA, we previously reported that a longstanding open ductus can affect cerebellar growth, using volume-based measurements. This growth impairment was independently and directly related to cerebral tissue oxygenation.34 Among premature neonates with a known hsPDA, it thus follows that rScO2 pattern monitoring, especially when demonstrating severe-range cerebral hypoxia, may impact treatment decisions regarding the need for PDA closure.34
Anemia
Numerous reports have demonstrated a negative association between anemia and cerebral oxygenation,35,36 and especially at hemoglobin values below 6 mmol/L.37 These same studies have additionally demonstrated improved cerebral and peripheral tissue oxygenation following packed red blood cell transfusion (Fig. 3c).49,50 Thus, proactive rScO2 monitoring may aid clinicians in determining the transfusion needs among premature neonates with already marginal hematocrits.
Hypoglycemia
During hypoglycemia, cerebral oxygenation increases, an insidious sign potentially indicative of a shortage in glucose and/or other substrates for cerebral metabolism and hence a redistribution of blood flow to the brain to enhance oxygen delivery.38 Whether rScO2 pattern monitoring can aid in hypoglycemia-related clinical management is yet to be elucidated, though the noninvasive nature of cerebral monitoring may provide value-addition at minimal risk.
PHVD
Finally, when progressive PHVD is diagnosed, increasing pressure exerted on the cerebral parenchyma by the expanding lateral ventricles can negatively impact cerebral tissue perfusion.39,51 Although not much is known about the consequences on tissue oxygenation, a recent study by Kochan et al.39 demonstrated rScO2 often below 40%, values which have been associated with cerebral impairment.43,44,45,46
Applying rScO2 “pattern recognition” in clinical practice?
As discussed above, observation of cerebral oxygenation patterns using NIRS-derived rScO2 and timely recognition of suboptimal patterns can potentially aid clinicians in avoiding various neonatal conditions associated with untoward cerebral consequences. Nurses, physician assistants, residents, and medical staff must be instructed according to practical guidelines concerning the interpretation of rScO2 patterns. Table 1 lists the most frequently occurring conditions, derived from the SafeboosC study,52 which may impact cerebral oxygenation and perfusion patterns. Applying rScO2 monitoring to clinical practice is predominantly a matter of discerning which condition(s) is/are most likely contributing to the changes in cerebral oxygen patterns for a given neonate.
In the University Medical Center Utrecht NICU, instruction sessions on physiologic basics and clinical use of cerebral NIRS are provided on a regular basis. During these sessions, “pattern recognition” with regard to cerebral oxygenation and/or extraction are discussed in interactive sessions. Interpretation of these patterns and possible interventional actions based on these interpretations are embedded within practical guidelines (see summary in Table 1) and reasonably implemented in the daily care of very and extremely preterm neonates. An e-learning program is available for the nurses and medical staff. All infants born at less than 30 weeks’ gestational age undergo rScO2 monitoring from NICU admission to 72 h of age, assuming this to be the most vulnerable period. Following 72 postnatal hours, cerebral oxygenation monitoring is continued in unstable infants remaining on the ventilator and/or those with an hsPDA. In any neonate, rScO2 monitoring may be restarted as needed, such as in the case of progressive PHVD. In all neonates, rScO2 monitoring is depicted in real-time at the bedside and is collected in the electronic medical record. Concomitant conventional monitoring is displayed alongside rScO2 data (e.g., mean arterial pressure). Additionally, offline analysis of rScO2 and conventional monitoring is possible using an in-house developed database (Signalbase®).
Issues to be solved
Establishing large-scale clinical application of NIRS-based cerebral oxygenation monitoring in the NICU requires international consensus among the neonatal community. Normative values and expected quiescent variability remain elusive data, which are critical to understanding cerebral oxygen utilization patterns among preterm neonates. Moreover, how these “normal” patterns are expected to change in response to single or multiple neonatal conditions remains an area of significant ongoing inquiry and controversy. In addition, though this review focuses solely on pattern recognition with regard to cerebral oxygenation monitoring, a growing body of work incorporates comparisons between cerebral and peripheral oxygenation measures.53 Ongoing research is required to determine how simultaneous multiregion monitoring may further clarify individual patients’ changes in clinical status. As we have described rScO2 effects related to individual conditions above, an individual patient’s physiology requires individual management, and certainly no set of cerebral oxygenation “rules” may apply to all neonates.
Legal implications
Also important for wide-scale utilization of rScO2 monitoring are the challenges and legal implications behind introducing new monitoring techniques. In a recent comprehensive review, Andersen et al. described the potential use of NIRS-derived monitoring of oxygenation adequacy as a factor in neonatal management.54 While this review focuses on cerebral oxygenation, peripheral forms of tissue oxygenation monitoring are also mentioned as potential contributors to an assessment of a given neonate’s overall physiologic oxygenation status. In addition, the introduction of a new monitoring strategy as a standard of care requires an eventual diminishing of equipoise concerning effects on outcomes, along with broad acceptance among clinicians and practitioners. At present, while rScO2 pattern monitoring shows promise for improved individualization of neonatal care and potentially earlier recognition of a variety of conditions associated with cerebral impairment, whether this monitoring ultimately impacts long-term outcomes is yet to be definitively proven.
Conclusion
Ongoing assessment and “pattern recognition” of NIRS-derived cerebral oxygen saturation (rScO2) seems a relatively simple modality for potentially preventing or reducing the incidence of severe PIVH and/or WMI in very and extremely preterm neonates. International collaboration and consensus are required to increase the utilization of this potentially neuroprotective strategy.
Change history
06 August 2018
The original version of this article contained an error in the legend of Fig. 3, which incorrectly read:
Figure 3. a The patterns of arterial saturation (SaO2; orange), and rScO2 (blue) and mean arterial blood pressure (MABP; red) of an extremely preterm infant on postnatal day 1. The initial rScO2 values were very low (red box). These low values seemed to be associated with PaCO2 values below 30 mmHg (brown squares; starting at 24 mmHg. SaO2 and MABPs values were always normal. When PaCO2 values increased above values of 30 mmHg (brown arrow) the rScO2 increased and eventually normalized. b The patterns of rScO2 (blue) and mean arterial blood pressure (MABP; red) of a very preterm girl, starting on postnatal day 1, was especially marked by a steep decrease in cerebral oxygenation (rScO2; red box) to very low values (<40%). Echocardiographic investigation early on postnatal day 2 revealed a hemodynamically significant ductus arteriosus. Subsequent ductal closure with indomethacin (2 courses) was followed by normalization of cerebral oxygenation. c The patterns of heart rate (HR), arterial saturation (SaO2) and rScO2 (red box) in a preterm neonate.with severe anemia. The rather low rScO2 recovered following packed red blood cell transfusion (courtesy Prof. Gunnar Naulaers, UZ Leuven).
This has been corrected in both the PDF and HTML versions of the article.
References
Van Bel, F., Lemmers, P. & Naulaers, G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology 94, 237–244 (2008).
Dix, L. M. L., van Bel, F. & Lemmers, P. M. A. Monitoring cerebral oxygenation in neonates: an update. Front. Pediatr. 5, 46 (2017).
Wintermark, P., Hansen, A., Warfield, S. K., Dukhovny, D. & Soul, J. S. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage 85, 287–293 (2014).
Alderliesten, T. et al. Brain oxygen saturation assessment in neonates using T2-prepared blood imaging of oxygen saturation and near-infrared spectroscopy. J. Cereb. Blood Flow Metab. 37, 902–913 (2016).
Tsuji, M. et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106, 625–632 (2000).
Brady, K. et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 41, 1951–1956 (2010).
Hyttel-Sorensen, S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomized clinical trial. BMJ 350, g7635 (2015).
Alderliesten, T. et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr. Res. 79, 55–64 (2016).
Plomgaard, A. M. et al. Early biomarkers of brain injury and cerebral hypo- and hyperoxia in the SafeBoosC II trial. PLoS ONE 12, e0173440 (2017).
Verhagen, E. A. et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev. Med. Child Neurol. 57, 449–455 (2015).
Sorensen, L. C. & Greisen, G. Precision of measurement of cerebral tissue oxygenation index using near-infrared spectroscopy in preterm neonates. J. Biomed. Opt. 11, 054005 (2006).
Greisen, G., Andrese, B., Plomgaard, A. M. & Hyttel-Sorensen, S. Cerebral oximetry in preterm infants: an agenda for research with a clear clinical goal. Neurophotonics 3, 031407–1–6 (2016).
Korcek, P., Stranak, Z., Sirc, J. & Naulaers, G. The role of near-infrared spectroscopy monitoring in preterm infants. J. Perinatol. 37, 1070–1077 (2017).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J. Neonatal Perinatal Med. 7, 89–100 (2014).
Dix, L. M., van Bel, F., Baerts, W. & Lemmers, P. M. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr. Res. 74, 557–563 (2013).
Vohr, B. R. et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics 105, 1216–1226 (2000).
Brown, N. C. et al. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J. Pediatr. 155, 32–38 (2009).
Volpe, J. J. Neurology of the Newborn 6th edn, 325–698 (Elsevier, Philadephia, PA, 2018).
Krediet, T. G., Kavelaars, A., Vreman, H. I., Heijnen, C. J. & van Bel, F. Respiratory distress syndrome-associated inflammation is related to early but not late peri/intraventricular hemorrhage in preterm infants. J. Pediatr. 148, 740–746 (2006).
Ballabh, P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 41, 47–67 (2014).
Alderliesten, T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698–704 (2013).
Perlman, J. M., McMenamin, J. B. & Volpe, J. J. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N. Engl. J. Med. 309, 204–209 (1983).
Dix, L. M. L. et al. Carbon dioxide fluctuations are associated with changes in cerebral oxygenation and electrical activity in infants born preterm. J. Pediatr. 187, 66–72.e1 (2017).
Bonestroo, H. J. C., Lemmers, P. M. A., Baerts, W. & van Bel, F. Effect of antihypotensive treatment on cerebral oxygenation of preterm infants without PDA. Pediatrics 128, e1502–e1510 (2011).
Alderliesten, T. et al. Hypotension in preterm neonates: low blood pressure alone does not affect neurodevelopmental outcome. J. Pediatr. 164, 986–991 (2014).
Inder, T. E., Wells, S. J., Mogridge, N. B., Spencer, C. & Volpe, J. J. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J. Pediatr. 143, 171–179 (2003).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet 8, 110–124 (2009).
Billiards, S. S. et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18, 153–160 (2008).
Brouwer, M. J. et al. brain injury on term-equivalent age MRI in relation to perinatal factors and neurodevelopmental outcome at two years. PLoS ONE 12, e0177128 (2017).
Favrais, G. et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 70, 550–565 (2011).
Shankaran, S. et al. Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia in low birth weight infants. Pediatrics 118, 1654–1659 (2006).
Hatzidaki, E. et al. Risk factors for periventricular leukomalacia. Acta Obstet. Gynecol. Scand. 88, 110–115 (2009).
Martin, C. G., Snider, A. R., Katz, S. M., Peabody, J. L. & Brady, J. P. Abnormal cerebral blood flow patterns in preterm infants with a large patent ductus arteriosus. J. Pediatr. 101, 587–593 (1982).
Lemmers, P. M. A. et al. Patent ductus arteriosus and brain volume. Pediatrics 137, e20153090 (2016).
Andersen, C. C., Karayil, S. M., Hodyl, N. A. & Stark, M. J. Early red cell transfusion favourably alters cerebral oxygen extraction in very preterm newborns. Arch. Dis. Child. Fetal Neonatal Ed. 100, F433–F435 (2015).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Monitoring regional tissue extraction in neonates 1250g helps identify transfusion tresholds independent of hematocrit. J. Neonatal Perinatal Med 7, 89–100 (2014).
Van Hoften, J. C., Verhagen, E. A., Keating, P., ter Horst, H. J. & Bos, A. F. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch. Dis. Child. Fetal Neonatal Ed. 95, F352–F358 (2010).
Vanderhaegen, J., Vanhaesebrouck, S., Vanhole, C., Casaer, P. & Naulaers, G. The effect of glycaemia on the cerebral oxygenation in very low birthweight infants as measured by near-infrared spectroscopy. Adv. Exp. Med. Biol. 662, 461–466 (2010).
Kochan, M. et al. Changes in cerebral oxygenation in preterm infants with progressive posthemorrhagic ventricular dilatation. Pediatr. Neurol. 73, 57–63 (2017).
Greissen, G. & Vannucci, R. C. Is periventricular leucomalacia a result of hypoxic-ischaemic injury? Hypocapnia and the preterm brain. Biol. Neonate 79, 194–200 (2001).
Benitz, W. E. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J. Pediatr. 30, 241–252 (2010).
Noori, S. Patent ductus arteriosus in the preterm infant: to treat or not to treat?. J. Perinatol. 30(Suppl.), S31–S37 (2010).
Hou, X. et al. Research on the relationship between brain anoxia at different regional oxygen saturations and brain damage using near-infrared spectroscopy. Physiol. Meas. 28, 1251–1265 (2007).
Kurth, C. D., McCann, J. C., Wu, J., Miles, L. & Loepke, A. W. Cerebral oxygen saturation-time threshold for hypoxic-ischemic injury in piglets. Anesth. Analg. 108, 1268–1277 (2009).
Dent, C. L. et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J. Thorac. Cardiovasc. Surg. 131, 190–197 (2006).
Kusaka, T. et al. Relationship between cerebral oxygenation and phosphorylation potential during secondary energy failure in hypoxic-ischemic newborn piglets. Pediatr. Res. 65, 317–322 (2009).
Limperopoulos, C. et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 116, 717–724 (2005).
Padilla, N., Alexandrou, G., Blennow, M., Lagercrantz, H. & Aden, U. Brain growth gains and losses in extremely preterm infanst at term. Cereb. Cortex 25, 1897–1905 (2015).
Dani, C., Pratesi, S., Fontanelli, G., Barp, J. & Bertini, G. Blood transfuisons increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 50, 1220–1226 (2010).
Sandal, G. et al. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion 54, 1100–1105 (2014).
Soul, J. S., Eichenwald, E., Walter, G., Volpe, J. J. & du Plessis, A. J. CSF removal in infantile posthemorrhagic hydrocephalus results in significant improvement in cerebral hemodynamics. Pediatr. Res. 55, 872–876 (2004).
Pellicer, A. et al. The SafeBoosC phase II randomized clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 104, 1271–1278 (2013).
Garvey, A. A. & Dempsey, E. M. Applications of near infrared spectroscopy in the neonate. Curr. Opin. Pediatr. 30, 209–215 (2018).
Andersen, C. C., Hodyl, N. A., Kirpalani, H. M. & Stark, M. J. A theoretical and practical approach to defining “adequate oxygenation” in the preterm newborn. Pediatrics 139, e20161117 (2017).
Acknowledgements
The authors thank Dr. Petra Lemmers and Dr. Willem Baerts for their critical reading of the manuscript and advice. No financial assistance was received in support of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Bel, F., Mintzer, J.P. Monitoring cerebral oxygenation of the immature brain: a neuroprotective strategy?. Pediatr Res 84, 159–164 (2018). https://doi.org/10.1038/s41390-018-0026-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0026-8
This article is cited by
-
Wearable fiber-free optical sensor for continuous monitoring of neonatal cerebral blood flow and oxygenation
Pediatric Research (2024)
-
Relationship of cerebral blood volume with arterial and venous flow velocities in extremely low-birth-weight infants
European Journal of Pediatrics (2023)
-
Biomarker und Neuromonitoring zur Entwicklungsprognose nach perinataler Hirnschädigung
Monatsschrift Kinderheilkunde (2022)
-
Changes in regional oxygen saturation of the kidney and brain of infants during hospitalization
Journal of Clinical Monitoring and Computing (2022)
-
Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions
Pediatric Research (2019)