Abstract
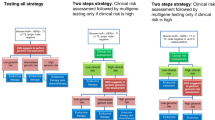
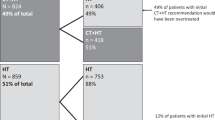
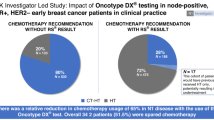
Gene expression profiling (GEP) testing using 12-gene recurrence score (RS) assay (EndoPredict®), 58-gene RS assay (Prosigna®), and 21-gene RS assay (Oncotype DX®) is available to aid in chemotherapy decision-making when traditional clinicopathological predictors are insufficient to accurately determine recurrence risk in women with axillary lymph node-negative, hormone receptor-positive, and human epidermal growth factor-receptor 2-negative early-stage breast cancer. We examined the cost-effectiveness of incorporating these assays into standard practice. A decision model was built to project lifetime clinical and economic consequences of different adjuvant treatment-guiding strategies. The model was parameterized using follow-up data from a secondary analysis of the Anastrozole or Tamoxifen Alone or Combined randomized trial, cost data (2017 Canadian dollars) from the London Regional Cancer Program (Canada) and secondary Canadian sources. The 12-gene, 58-gene, and 21-gene RS assays were associated with cost-effectiveness ratios of $36,274, $48,525, and $74,911/quality-adjusted life year (QALY) gained and resulted in total gains of 379, 284.3, and 189.5 QALYs/year and total budgets of $12.9, $14.2, and $16.6 million/year, respectively. The total expected-value of perfect information about GEP assays’ utility was $10.4 million/year. GEP testing using any of these assays is likely clinically and economically attractive. The 12-gene and 58-gene RS assays may improve the cost-effectiveness of GEP testing and offer higher value for money, although prospective evidence is still needed. Comparative field evaluations of GEP assays in real-world practice are associated with a large societal benefit and warranted to determine the optimal and most cost-effective assay for routine use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian cancer statistics 2017. Toronto, ON: Canadian Cancer Society; 2017.
Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M. Trends in breast cancer by race and ethnicity. CA Cancer J Clin. 2003;53:342–55.
Members of the Breast Cancer Disease Site Group. Surgical management of early-stage invasive breast cancer. In: Brackstone M, Tey R, reviewers. Program in evidence-based care evidence-based series no.: 1-1 Version 3. Education and Information 2015. Toronto, ON: Cancer Care Ontario; 2011. Endorsed 19 Nov 2010.
Eisen A, Fletcher GG, Gandhi S, Mates M, Freedman OC, Dent SF, et al. Optimal systematic therapy for early female breast cancer. In: Program in evidence-based care evidence-based series no.: 1–21. Toronto, ON: Cancer Care Ontario; 2014.
Early Breast Cancer Trialists' Collaborative Group, Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44.
Gyorffy B, Hatzis C, Sanft T, Hofstatter E, Aktas B, Pusztai L. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res. 2015;17:11.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34.
Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–83.
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65.
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–34.
Ioannidis JP. Is molecular profiling ready for use in clinical decision making? Oncologist. 2007;12:301–11.
Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, Cowens JW, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–11.
Kos Z, Nielsen TO. Developing a new generation of breast cancer clinical gene expression tests. Breast Cancer Res. 2014;16:103.
Michiels S, Ternes N, Rotolo F. Statistical controversies in clinical research: prognostic gene signatures are not (yet) useful in clinical practice. Ann Oncol. 2016;27:2160–7.
Hannouf MB, Xie B, Brackstone M, Zaric GS. Cost-effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in women with early-stage estrogen- or progesterone-receptor-positive, axillary lymph-node negative breast cancer. BMC Cancer. 2012;12:447.
Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard KI. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist. 2010;15:457–65.
Chang MC, Souter LH, Kamel-Reid S, Rutherford M, Bedard P, Trudeau M, et al. Clinical utility of multigene profiling assays in early-stage breast cancer. In: Program in evidence-based care recommendation report no.: MOAC-4. Toronto, ON: Cancer Care Ontario. Available on the CCO website: www.cancercare.on.ca/toolbox/qualityguidelines/clin-program/pathlabebs/pebc_moleconc/. Accessed 10 July 2017.
Buus R, Sestak I, Kronenwett R, Denkert C, Dubsky P, Krappmann K, et al. Comparison of EndoPredict and EPclin with oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016;108.https://doi.org/10.1093/jnci/djw149.
Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–90.
Sestak I, Buus R, Cuzick J, Dubsky P, Kronenwett R, Denkert C, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:545–53.
Inadomi JM. Decision analysis and economic modelling: a primer. Eur J Gastroenterol Hepatol. 2004;16:535–42.
Younis T, Rayson D, Thompson K. Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer. 2012;20:2523–30.
Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Mortality, leukemic risk, and cardiovascular toxicity of adjuvant anthracycline and taxane chemotherapy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;135:335–46.
Guidelines for the economic evaluation of health technologies. 4th ed. Ottawa, Canada: CADTH; 2017.
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38.
Levine MN, Julian JA, Bedard PL, Eisen A, Trudeau ME, Higgins B, et al. Prospective evaluation of the 21-gene recurrence score assay for breast cancer decision-making in Ontario. J Clin Oncol. 2016;34:1065–71.
Cancer Care Ontario. Cancer Care Ontario GCSF recommendations 2016. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=352101. Accessed 24 July 2017.
Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–62.
Kirwan CC, McDowell G, McCollum CN, Byrne GJ. Incidence of venous thromboembolism during chemotherapy for breast cancer: impact on cancer outcome. Anticancer Res. 2011;31:2383–8.
Wolff AC, Blackford AL, Visvanathan K, Rugo HS, Moy B, Goldstein LJ, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33:340–8.
Statistics Canada/Health Statistics Division. Life Tables, Canada and the Provinces, 2000–2002. Ottawa, Ontario, Canada: Minister of Industry; 2006. Publication 84-537-XIE.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41.
Ministry of Health and Long‐Term Care. Out‐of‐Country Program. Kingston, ON, Canada: Ministry of Health and Long‐Term Care; 2016.
Prosigna [Package Insert]. Seattle, WA: NanoString Technologies, Inc. Available at http://www.lifelabsgenetics.com/breast-cancer-prosigna-assay/paymentinformation. Accessed 18 June 2017.
British Colombia Cancer Agency. Laboratory services, oncotype Dx testing and Prosigna testing. BC, Canada. Available at http://www.bccancer.bc.ca/health-professionals/clinical-resources/laboratory-services. Accessed 13 Nov 2017.
Alberta Health Services. Laboratory services, breast cancer molecular testing. AB, Canada: Oncologist. Available at https://www.albertahealthservices.ca/assets/wf/lab/wf-lab-breast-cancer-molecular-testing-oncologist.pdf. Accessed 13 Nov 2017.
Bank of Canada. Home>Rates and Statistics>Related Information>Inflation Calculator [Web resource]. Ottawa, ON: Bank of Canada. Available at www.bankofcanada.ca/en/rates/inflationcalc.html. Accessed 20 June 2013.
Statistics Canada, National Center for Health Statistics, Centers for Disease Control and Prevention: Joint Canada/United States Survey of Health, 2002–03; 2004.
McKenna C, Claxton K. Addressing adoption and research design decisions simultaneously: the role of value of sample information analysis. Med Decis Making. 2011;31:853–65.
Laupacis A, Feeny D, Detsky AS, Tugwell PX. Tentative guidelines for using clinical and economic evaluations revisited. CMAJ. 1993;148:927–9.
Laupacis A. Economic evaluations in the Canadian common drug review. Pharmacoeconomics. 2006;24:1157–62.
Sparano JA. TAILORx: trial assigning individualized options for treatment (Rx). Clin Breast Cancer. 2006;7:347–50.
Hayes DF. Targeting adjuvant chemotherapy: a good idea that needs to be proven! J Clin Oncol. 2012;30:1264–7.
Bartlett J, Canney P, Campbell A, Cameron D, Donovan J, Dunn J, et al. Selecting breast cancer patients for chemotherapy: the opening of the UK OPTIMA trial. Clin Oncol. 2013;25:109–16.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–14.
Butts C, Kamel-Reid S, Batist G, Chia S, Blanke C, Moore M, et al. Benefits, issues, and recommendations for personalized medicine in oncology in Canada. Curr Oncol. 2013;20:e475–83.
Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011;17:6012–20.
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7.
Tang G, Cuzick J, Costantino JP, Dowsett M, Forbes JF, Crager M, et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol. 2011;29:4365–72.
Duffy MJ, Harbeck N, Nap M, Molina R, Nicolini A, Senkus E, et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer. 2017;75:284–98.
National Institute for Health and Care Excellence. Overview—tumour profiling tests to guide adjuvant chemotherapy decisions in people with breast cancer (update of DG10). Issue date: November 2017.
van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
Cardoso F, van't Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–29.
Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making. 2003;23:341–50.
Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE, Jacobs SA, et al. Interim joint analysis of the ABC (anthracyclines in early breast cancer) phase III trials (USOR 06-090, NSABP B-46I/USOR 07132, NSABP B-49 [NRG Oncology]) comparing docetaxel+cyclophosphamide (TC) v anthracycline/taxane-based chemotherapy regimens (TaxAC) in women with high-risk, HER2-negative breast cancer. J Clin Oncol. 2016;34.
Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–83.
Ontario Ministry of Health and Long-Term Care. Ontario Health Insurance (OHIP) schedule of benefits and fees; 2007. http://www.health.gov.on.ca/english/providers/program/ohip/sob/sobmn.html. Accessed 13 Aug 2017.
Mittmann N, Verma S, Koo M, Alloul K, Trudeau M. Cost effectiveness of tac versus fac in adjuvant treatment of node-positive breast cancer. Curr Oncol. 2010;17:7–16.
Lathia N, Isogai PK, De Angelis C, Smith TJ, Cheung M, Mittmann N, et al. Cost-effectiveness of filgrastim and pegfilgrastim as primary prophylaxis against febrile neutropenia in lymphoma patients. J Natl Cancer Inst. 2013;105:1078–85.
Skedgel C, Rayson D, Younis T. The cost-utility of sequential adjuvant trastuzumab in women with Her2/Neu-positive breast cancer: an analysis based on updated results from the HERA Trial. Value Health. 2009;12:641–8.
Guanella R, Ducruet T, Johri M, Miron MJ, Roussin A, Desmarais S, et al. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: a prospective evaluation. J Thromb Haemost. 2011;9:2397–405.
Wehler E, Zhao Z, Pinar Bilir S, Munakata J, Barber B. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur J Health Econ. 2017;18:49–58.
Levy AR, Zou D, Risebrough N, Buckstein R, Kim T, Brereton N. Cost-effectiveness in Canada of azacitidine for the treatment of higher-risk myelodysplastic syndromes. Curr Oncol. 2014;21:e29–40.
Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert Rev Pharm Outcomes Res. 2010;10:553–66.
Lucioni C, Finelli C, Mazzi S, Oliva EN. Costs and quality of life in patients with myelodysplastic syndromes. Am J Blood Res. 2013;3:246–59.
Enden T, Wik HS, Kvam AK, Haig Y, Klow NE, Sandset PM. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study. BMJ Open. 2013;3:e002984.
Acknowledgements
The ATAC trial investigators provided some summary statistical estimates for this research. The results and conclusions are those of the authors, and no official endorsement by ATAC trial investigators is intended or should be inferred.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hannouf, M.B., Zaric, G.S., Blanchette, P. et al. Cost-effectiveness analysis of multigene expression profiling assays to guide adjuvant therapy decisions in women with invasive early-stage breast cancer. Pharmacogenomics J 20, 27–46 (2020). https://doi.org/10.1038/s41397-019-0089-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-019-0089-x
This article is cited by
-
Performance of a novel spectroscopy-based tool for adjuvant therapy decision-making in hormone receptor-positive breast cancer: a validation study
Breast Cancer Research and Treatment (2024)
-
PROCURE European consensus on breast cancer multigene signatures in early breast cancer management
npj Breast Cancer (2023)
-
PAM50 intrinsic subtypes, risk of recurrence score and breast cancer survival in HIV-positive and HIV-negative patients—a South African cohort study
Breast Cancer Research and Treatment (2023)
-
The beneficial role of Asian-based RecurIndex test in the prognostic prediction in Chinese male breast cancer patients
Scientific Reports (2021)
-
Analysis of extracellular vesicle mRNA derived from plasma using the nCounter platform
Scientific Reports (2021)