Abstract
Exosomes are a class of extracellular vesicles of endocytic origin, which are released by cells and are accessible in biofluids, such as saliva, urine, and plasma. These vesicles are enriched with small RNA, and they play a role in many physiological processes. In the brain, they are involved in processes including synaptic plasticity, neuronal stress response, cell-to-cell communication and neurogenesis. While exosomes have been implicated previously in cancer and neurodegenerative diseases, research regarding their role in mental disorders remains scarce. Given their functional significance in the brain, investigation in this field is warranted. Additionally, because exosomes can cross the blood–brain barrier, they may serve as accessible biomarkers of neural dysfunction. Studying exosomes may provide information towards diagnosis and therapeutic intervention, and specifically those derived from the brain may provide a mechanistic view of the disease phenotype. This review will discuss the roles of exosomes in the brain, and relate novel findings to current insights into mental disorders.
Similar content being viewed by others
Introduction
There has been growing interest in the development of personalized approaches in psychiatry over the last decade. Part of this drive is based on the fact that mental disorders are etiologically heterogeneous, and treatments, while effective, are helpful only in a portion of patients1. Additionally, patient treatment response is difficult to predict. As a result, there has been much interest in the discovery of biomarkers which, if successful, could assist clinicians in the determination of personalized treatment strategies. Biomarker research is largely based on the investigation of peripheral tissues, particularly when focused on the study of molecular markers. The relationship of peripheral findings to events taking place in the central nervous system (CNS) is an important limitation of these studies. Thus, much enthusiasm has been generated by advances in exosome research. These small extracellular vesicles are released by cells, carry molecular signals, and are involved in cellular communication2,3. Additionally, they can cross the blood–brain barrier (BBB), and can be detected peripherally, making them intriguing candidates in mental health biomarker discovery2,4.
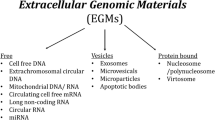
The term “extracellular vesicles” (EVs) encompasses a group of cell-derived vesicles produced by most, if not all cell types, that are released to the extracellular environment3. Growing evidence suggests that these vesicles have a functional impact on physiological processes, and are especially vital in cell-to-cell communication2,3. Since the EV field has grown, different types of vesicles have been described, which differ in their properties, as well as their biogenesis (Fig. 1)3. The three main types are: apoptotic bodies (500–2000 nm), microvesicles (50–1000 nm), and exosomes (40–200 nm)5. Apoptotic bodies are EVs that bud off the membrane of cells undergoing apoptosis, and are typically engulfed by macrophages3. Microvesicles directly bud off the plasma membrane and contain a range of cargo that is delivered to neighbouring cells3. Exosomes, which are the smallest class of extracellular vesicles, first develop as intraluminal vesicles (ILV) through the inward budding of the multivesicular body (MVB)3,6. The MVB has two potential fates; it can either fuse with the lysosome, leading to the degradation of its contents, or fuse with the plasma membrane, and release its ILV contents as exosomes into the extracellular space (Fig. 1)3. In terms of cargo, exosomes contain a variety of biological materials including proteins, lipids, and nucleic acids3. Notably, compared to plasma, saliva, or other biological fluids, exosomes are highly enriched in microRNA (miRNA)5,7. The majority of miRNA that can be accessed from serum or saliva are contained in exosomes, and some miRNA appear to be dependent on exosomes as they go undetected as free floating molecules in biofluids7. Although there is currently no concrete evidence to show there is a miRNA sorting mechanism for exosomes, there is evidence to suggest that this is a possibility. MiRNA profiles of exosomes do not always match the profiles of parent cells, and observations of miRNA enrichment in exosomes further suggests a mechanism of selective miRNA export8,9. Additionally, miRNA expression in exosomes can be altered based on physiological changes such as disease state, making the miRNA cargo intriguing candidates for investigation. To date, there is evidence of altered exosomal cargo in disease development and progression in pathologies such as cancer10 and neurodegenerative diseases11,12. However, to date there is only one study that has investigated exosomal miRNA cargo alterations in mental disorders13. Banigan et al.13 used exosomes from frozen postmortem prefrontal cortex to study miRNA alterations in schizophrenia and bipolar disorder13. They found that miR-497 in schizophrenia patients and miR-29c in bipolar patients to be upregulated compared to controls13. This early work opens up interesting possibilities for the study of exosomes in mental disorders, demonstrating that miRNA cargo may be interesting to investigate in these phenotypes. Indeed, miRNAs have already been implicated in several mental disorders, such as depression, schizophrenia, anxiety, and bipolar disorder, including being implicated as candidate peripheral biomarkers for disease development and treatment response14,15,16,17.
Apoptotic bodies, the largest of the EVs, “bleb” off the cell membrane and contain material from cells undergoing apoptosis to signal to macrophages. Microvesicles bud off the plasma membrane and contain cargo that can facilitate signaling to recipient cells. Exosomes are the smallest of the vesicles, and are first made as a population of heterogeneous intraluminal vesicles in the multivesicular body (MVB). The MVB has two fates, either fusing with the lysosome, or fusing with the plasma membrane where they are released as exosomes. Exosomes can be taken up by other cells either by endocytosis, micropinocytosis, or phagocytosis where its contents can effectively influence cellular processes. Contents can be involved in transcriptional regulation, or mRNA cargo can be transcribed in recipient cells
In recent years, efforts to characterize exosomal release and uptake have had important implication for their role in the CNS. Previous studies have demonstrated that exosomes and their cargo play a role in normal communication in the CNS, as well as nerve regeneration, synaptic function, plasticity, and immune response18,19,20. In addition to their critical role in normal brain function, exosomes have also been implicated in the propagation of neurodegenerative diseases12,21. Given exosomes’ role in normal brain physiology, and their contribution to other CNS disease states such Parkinson’s12, and Alzheimer’s21 it is reasonable to hypothesize exosomes may play a significant role in the pathogenesis of mental disorders. Exosomes have been found to play a role in processes that have long been hypothesized to be involved in psychopathology of mental disorders, such as neuroinflammation22, neurogenesis23, plasticity24,25, and epigenetic regulation26. Additionally, their ability to cross the blood–brain barrier (BBB) suggests that exosome content in the CSF and plasma may reflect ongoing neural processes27. Therefore, information from neural-derived exosomes found in peripheral sources might be able to provide relatively non-invasive markers of clinical utility for mental disorders.
This review highlights the role of exosomes in the CNS. Particularly, it focuses on their role and cargo in the brain; their ability to cross the blood–brain barrier; and their release and transfer. Recent insights in the properties of exosome signaling in the brain are then related to existing pathophysiological perspectives of mental disorders. Results discussed here support the notion that the function of exosomes in the brain may align with neurobiological theories of mental disorders, and that exosomes have the potential to be strong biomarker candidates for this psychopathology.
Cell communication via exosomes in the brain
Exosomes play important roles in cell communication in the CNS, acting on both neighbouring and distal cells (Fig. 2)28. These vesicles can act as important vehicles of communication both within a cell type, and between different cell types. Evidence from multiple studies demonstrates that exosome release from cells in the CNS is a highly regulated process, with release regulated by synaptic glutamatergic activity and calcium influx18,29. Neuronal exosome release is triggered by Ca2+ entry through N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors at glutamatergic synapses, suggesting that exosome release may be part of normal synaptic physiology18. Additionally, the controlled subcellular location of release of exosome has been documented in neurons; however, the mechanism is unclear30. MVBs are ~50 times more represented in soma or dendrite compartments compared to axons30. Although the mechanism for preferred compartmentalization is unknown, these areas of specific enrichment further support both, a role for exosomes at synapses, and their highly regulated release.
Exosome signalling is involved in many physiological brain processes. Changes in many of these processes have been previously associated in mental disorders. For example, activated monocytes release exosomes that can influence BBB permeability. A leaky BBB is associated with neuroinflammation, and has been previously implicated in schizophrenia, bipolar disorder, and major depressive disorder. Additionally, exosomes carry markers from parent cells that may help them be distinguishable in biofluids
Serotonin (5-HT) has also been implicated in the release of exosomes from non-neuronal cells types in the brain. Serotonin can increase cytosolic levels of calcium, which in turn, stimulates the release of exosomes from primary microglial cells29. Dysregulation in serotonin pathways has been implicated in depression, anxiety, bipolar disorder, and schizophrenia, with its receptors being targets of some of the most commonly prescribed drugs31,32,33,34. Given that microglial release of exosomes can be regulated by serotonin, and serotonin is often found to be altered in mental disorders, it follows that microglial exosome release may also be modified in these disorders. Both neurotransmitter release and cell communication are important factors in psychopathology35. It will therefore be important to understand the role exosomes may play in the etiopathogenesis of mental disorders given their prominence in the regulation of cell communication, and their regulation via neurotransmitters.
Basic neuron-to-neuron communication can occur through exosome release and uptake36. Strikingly, it was demonstrated that a subpopulation of neuron-internalized exosomes can be re-secreted along with the recipient neuron’s endogenous exosomes, seemingly to facilitate long-distance interactions36. While the eventual fate of these exosomes remains undetermined, these findings demonstrate the ability of exosomes to mediate communication within a cell type and the potential for widespread signaling36. Additionally, neuron-to-neuron signalling via exosomes has found to be involved in important processes including synaptic plasticity (Fig. 2)37.
Exosomes are also mediators of cellular communication between cell types, with evidence of glial-to-neuron communication38. In a feedback loop-like manner, neurotransmitter release can stimulate oligodendrocyte exosome secretion, while neurons are able to internalize oligodendrocyte exosomes and utilize their cargo39. The internalization of oligodendrocyte-derived exosomes by neurons can result in greater tolerance to stress and increased viability resulting in a form of cellular protection (Fig. 2)39. Additionally, neuron to microglial communication also occurs via exosomes (Fig. 2). When neurons were co-cultured with microglial cells, neuron-derived exosomes were internalized by the microglial cells19. This internalization resulted in an enhancement of the cells removing degenerative neurites19.
Although knowledge regarding astrocyte-neuron communication via exosomes remains scarce, there is evidence to suggest that it does occur, and this method of communication is critical for neuronal cell survival (Fig. 2). Prion protein (PrP) is an important protective protein against oxidative stress. Protection of neurons via astrocyte-derived exosomes was dependent on astrocyte-derived exosomal PrP transportation into neurons40. Considering all of these reports together, cell communication via exosomes is emerging as an important regulator of neuron protection and synaptic plasticity (Fig. 2), and their dysregulation has been implicated in the pathophysiology of mental disorders, such as bipolar disorder, major depressive disorder, and schizophrenia41,42,43,44. Neuroprotective signaling is required for proper growth and survival of neurons, and alterations in the number neurons, glia, and neuropils have previously been reported in mental disorders42,43.
After release into the extracellular space, exosomes can be internalized by recipient cells via several mechanisms including phagocytosis, micropinocytosis, endocytosis, and plasma membrane fusion (Fig. 1.)45. Most investigations of exosomes in the CNS report endocytosis-based uptake46,47 but, there is also evidence to suggest that uptake depends on the recipient cell type. Specifically, it was found that exosomes released from neuroblastoma cells are preferentially endocytosed by glial cells, whereas exosomes released from cortical neurons are selectively endocytosed by other neurons48.
Once exosomes have been internalized by recipient cells, their exosome cargo may elicit an effect on the cell (Fig. 1)9. One of the first studies to demonstrate functional exosome cargo transfer to recipient cells was done by Valadi and colleagues in 20079. After incubation and transfer of mouse exosomes to human cells, three new mouse proteins were found in recipient human cells, therefore providing evidence that exosome mRNA can be translated in recipient cells9.
Since the original discovery that exosome cargo transfer displays functional effects in the recipient cell, there have been several studies investigating this mechanism as a means of cellular communication in both disease and healthy states. For example, an exosome’s ability to spread cargo and elicit an effect on recipient cells has been identified as a potential pathway involved in cancer development and progression. Exosomes isolated from colon cancer cells expressing a mutant form of the protein K-RAS (KRAS) contain the mutant KRAS along with numerous proteins that have the ability to promote tumor progression49. These exosomes, which can be internalized by wild-type colon cells, can transfer the mutant protein to healthy cells, effectively leading to enhanced growth of these cells49. In the brain, communication via exosomes has been found to play a role in Alzheimer’s disease progression by neuron-to neuron transport of misfolded amyloid-beta oligomers50. Using an in vitro model, exosome formation and secretion was blocked via the siRNA knockdown of proteins required for these functions, and the spread of the oligomers was in turn also blocked50. Although there are clear phenotypic and mechanistic differences between cancer, Alzheimer’s, and mental disorders, and given the continuum in which mental disorders lie, we would expect quantitative and not dichotomous changes in this phenotype. Nonetheless, results from the studies above show that exosomes can propagate disease spreading through cargo transfer. Since miRNAs have previously been implicated mental disorders14,15,16,17, it is possible that exosome miRNA transfer is contributing to the progression of phenotype, as well as individual symptoms. It would be of interest to investigate whether miRNA associated with psychiatric phenotypes are packed into exosomes, and whether these exosomal miRNA profiles are altered in mental disorders.
The ability of exosomes to cross the blood–brain barrier (BBB)
The BBB lies at the interface of the peripheral circulatory system and the CNS, acting as a highly selective membrane that protects the brain’s microenvironment and preserves homeostasis51. The BBB is mainly comprised of brain macrovascular endothelial cells (BMECs) and tight junctions to prevent the transfer of potentially toxic compounds between the blood and the brain52. Besides transmembrane diffusion of small (<400 Da) lipid soluble molecules, the BBB allows for selective transport of some compounds into and out of the brain52. Transport of material across the BBB can be either transcellular through BMECs, or paracellular through junctions between BMECs53.
The findings that exosomes can cross the BBB, and that its contents remain active, have been instrumental in biomarker research with exosomes and their use as a drug delivery system. Alvarez-Erviti et al. demonstrated effective delivery of siRNA to the brain via systemic injection of exosomes in mice26. They engineered dendritic cells to express lysosome-associated membrane protein 2 (Lamp2b), an exosomal membrane protein26. By fusing Lamp2b to a rabies virus glycoprotein (RVG) peptide that is specific to the CNS, the exosomes were targeted exclusively to the brain26. These exosomes delivered GAPDH siRNA, which resulted in specific gene knockdown exclusively in the brain26. Later studies have been successful in delivering exosomes via intranasal injection in mice to the brain54. Most recently, a study using rats identified that a fluorescently tagged protein expressed selectively in brain tissue could be recovered in small EVs (those with the same characteristics as exosomes) in their blood4. This study provides evidence of communication via exosomes from the brain to the rest of the body4. Evidence from these studies support the notion that exosomes cross the BBB in a bi-directional manner; however, their exact method of crossing remains unclear.
Much of the current research examining how exosomes can cross the BBB points to the transcellular method of transport through BMECs via the different mechanisms of endocytosis. The transfer of EVs derived from human erythrocytes in an in vitro BBB model was dependent on the adsorptive-mediated transcytosis method of transport55. Although the EVs did cross under healthy and inflammatory conditions, EVs movement across the BBB was significantly higher after the peripheral administration of lipopolysaccharides55. Another study by Chen et al.53 demonstrated exosomes crossing a BBB model using transcellular BMEC endocytosis in healthy and stroke-like condition, suggesting that exosomes retain their ability to cross during stressful states53. This group demonstrated that exosomes are internalized through endocytosis, and accumulate in endosomes. After MVB formation, the exosomes are then released on the other side of the BMEC monolayer. The data suggest that exosomes derived from human embryonic kidney cells could cross using multiple pathways of endocytosis53. Using an inhibitor for clathrin-dependent endocytosis, chlorpromazine (CPZ), which transfers clathrin from the surface of cells to intracellular endosomes56, there was a decrease in exosome transcellular migration53. This suggests that clathrin-dependent endocytosis may be involved in transportation of exosomes across the BBB53. Additionally, methyl-β-cyclodextrin (MβCD), which removes cholesterol from the plasma membrane56, and filipin III, which binds to cholesterol56, also significantly reduced exosomes crossing the BBB53. This result suggests that caveolae-dependent endocytosis is another possible route of migration. It is rather likely that uptake of exosomes in BMEC will depend on specific ligand receptors or lipid rafts, and mechanisms of exosome uptake may depend on the cell of origin. Exosomes from different cells may have different cargo including protein and lipids, potentially altering their method of crossing the BBB57. Furthermore, disease state may impact the method used to cross the BBB, as cargo can change upon disease state13,57.
In addition to crossing, recent studies have elucidated a role for exosomes in increased permeability of vascular barriers of the BBB. For example, exosomes secreted from breast cancer cells uniquely express miR-105, which directly targets the tight junction protein ZO-158. This exosome transfer of miR-105 destroys tight junctions and the integrity of the BBB58. In addition, claudin-5 (Cldn5) has been found to be encapsulated in exosomes, which is a tight junction protein present in the BBB59. When Cldn5 is knocked out in mice, it results in the loosening of the BBB60, suggesting that exosomes carrying Cldn5 may play a role in BBB integrity. Interestingly, a decrease in Cldn5 is sufficient to induce depressive-like behaviors in these mice, and treatment with antidepressants increases Cldn5 levels and promotes disease resilience61. A leaky BBB is associated with neuroinflammation—a prominent theory of mental disorders62,63. Therefore, the possibility of exosomes influencing the integrity of the BBB may also suggest a role for exosomes in neuroinflammation and the pathogenesis of mental disorders63. Taking it one step further, a leaky BBB state in mental disorders may be initiated by exosomes released from cells being influenced by this disease state.
Crossing the BBB allows for communication between the periphery and the CNS, therefore communication via exosomes may account for some of the systemic changes observed in several mental disorders. For example, the bi-directional communication between the gut microbiome and the brain has previously been associated with mental disorders, with most attention focusing on its link to depression64,65. Additionally, dysregulation of systemic immune response has been well documented in mental disorders including depression, schizophrenia, and bipolar disorder66. It is possible that cells responding to a psychiatric state may release exosomes that, in turn, affect the peripheral inflammatory response or the gut microbiome. In addition to exosomes being a link between the CNS and periphery in mental disorders, peripheral access to CNS-derived vesicles make them ideal carriers of potential biomarkers. These vesicles are better suited to providing insight into changing mechanisms in the CNS of affected individuals.
Since the discovery of exosomes’ ability to cross the BBB, there has been increasing interest in their ability to act as a drug delivery system to the brain. They have been found to be a promising vehicle for drug delivery in many types of cancers, both in vivo and in vitro67 showing they are able to deliver drugs across the BBB. In cancer, reports have shown that delivery of drugs across the BBB resulted in decreased markers for brain tumor growth68. Other than cancer, exosomes have been found to be an effective drug delivery system for brain-related diseases. A formulation of catalase, a promising treatment for Parkinson’s disease, can be loaded into exosomes and reach target neurons where the drug then accumulates69. In a study by Liu et al., exosomes expressing neuron-specific rabies viral glycoprotein (RVG) peptide were used to deliver opioid receptor mu (MOR) siRNA into the brain to treat morphine addiction70. The exosomes efficiently delivered the MOR siRNA into the mouse brain and reduced MOR, resulting in the inhibition of morphine relapse70. Their role as a drug delivery system seems extremely promising, and this could be an interesting line of research for further investigation, as there are many advantages to implementing targeted treatment in mental disorders. Using nanotechnology for drug delivery to the brain has the potential to alleviate some of the peripheral symptoms in mental disorders, as well as solve the problem of delivery across the BBB and drug solubility71.
Exosome biogenesis in disease states
Exosomes were once thought to be a rather homogenous population of vesicles; however, more recently, studies have found that they are rather diverse72. Exosome biogenesis appears to be a more dynamic process, with heterogeneous populations of exosomes being produced. A study by Willms et al.72 identified a large (75–200 nm in size) and small (most <100 nm) population of exosomes from the same cell type72. They repeated this experiment with different cell types, as well as plasma, and found similar results72. Results suggested that the two different populations had distinct protein and RNA profiles72. In the smaller exosomes they identified less individual proteins (110 proteins compared to 254 in larger vesicles), suggesting the smaller vesicles had more specific types of protein cargo72. Additionally, the smaller vesicles were enriched in smaller RNA molecules compared to the larger vesicles72. Although currently there is no evidence for roles of the different sized exosomes, it would not be surprising if smaller exosomes contained less, or smaller material as briefly eluded above72. This study used nanoparticle tracking analysis (NTA) to characterize exosomes by size; however, there are multiple technologies that can be used for this measurement. Particle size profiling and/or quantification can also be measured using technologies including tunable resistive pulse sensing73, high resolution flow cytometry74, and optical disc technology75. Consistencies in technologies is imperative as each technology may yield different results from the same sample76.
Although there is not much evidence in changes in size given a disease state, there is evidence to suggest that biogenesis is affected in disease states as exosome quantity may be altered. Because the field of exosomes in mental disorders is in its infancy, changes in exosome biogenesis have yet to be studied thoroughly. Exosome biogenesis seems to be enhanced in cancer, with tumor cells secrete more exosomes than non-tumor cells, and exosome levels of cancer patients are often elevated77. In one specific investigation, quantification of exosomes from plasma showed that esophageal cancer patients expressed higher exosome levels compared to patients with a non-malignant tumour78. Another study used plasma from patients with ovarian cancer and found similar results79. Subjects with malignant tumours had more exosomes than those with benign79. And subjects in the malignant and benign groups had more exosomes than healthy controls79. Escalating amounts of exosomes may result in an increase in signalling between cells. Additionally, altered cargo in these vesicles may aid in tumor and disease progression.
Although most of the research on changes in exosome biogenesis has been conducted in cancer, this is some evidence to show that these changes may occur in other disease states affecting the brain. Enriched exosome secretion is documented in brains of individuals with Down syndrome, and a knockdown of exosome secretion resulted in worsening endosomal pathology in fibroblasts from these patients80. Additionally, an increase in EV-associated protein, suggesting an increase in EVs, was observed in serum from subjects with autism spectrum disorder (ASD)81. Results from a study in 2017 showed that individuals with HIV had less exosomes in plasma than healthy controls82. Neurological deficits, HIV-associated neurocognitive disorder (HANDS), develops in a portion of adults with HIV82. Patients that were neuropsychologically impaired had fewer neuron-derived exosomes than patients who were neuropsychologically normal82. Fewer exosomes may result in a change or lack of signaling between cells. Results from the above studies suggest that exosomes are extremely heterogeneous in nature and that biogenesis can be altered in disease states. Investigation into exosome biogenesis may provide more insight into the etiology of mental disorders. Identifications of altered amounts or sizes in mental disorders may provide more insight into changes in cellular communication occurring within the disease state.
Possible role of exosomes in the pathogenesis of mental disorders
Current evidence for exosome signaling in the brain points toward their role in transcriptional regulation83, neurogenesis23, plasticity24,25, and neuroinflammation22,62. Changes in these mechanisms have also been previously implicated in mental disorders, providing reason to hypothesize that exosomes may be involved in these phenotypes.
Neurogenesis has been previously implicated in schizophrenia and depression, and research suggests that these disorders are associated with impaired adult hippocampal neurogenesis (AHN)23. Protein analysis of exosomes in the CNS reveals cargo involved in modulating adult neurogenesis23. Furthermore, the injection of cultured exosomes containing known pathogens into the dentate gyrus is sufficient to impair AHN in mice84. CSF-derived factors and substances such as corticosteroids and cytokines may trigger the release of astrocytic exosomes containing several miRNAs important for neurogenesis, stress response, and cell survival23. Thus, it is possible that exosomes are involved in both the maintenance and hindrance of adult neurogenesis.
Protein analyses of exosomes in the CNS reveal that some cargo is involved in modulating synaptic plasticity, suggesting exosomes may play a role in this process24,25. For example, microtubule-associated protein 1B (MAP1b), a protein associated with synaptic plasticity, was identified in exosomes from depolarized human neurons in culture85. When microglial cells were incubated with neuron-derived exosomes, removal of neurites was accelerated by increasing the expression of complement component 3 (C3) in the microglial cells19. Neuron-to-glial signalling via exosomes is one mechanism where active synapses stimulate the pruning of those that are inactive, thereby promoting synaptic plasticity19.
There is also mounting evidence for the role of exosomes in neuroinflammation. Upon exposure to the pro-inflammatory cytokine tumour necrosis factor (TNF), exosomal protein cargo from brain endothelial cells is altered86. These exosomes contained proteins involved in TNF and NF-ĸB signaling pathways86. The neuroinflammation caused by TNF relates to the low-level, chronic neuroinflammation associated with certain forms of psychopathology, particularly depression62. Additionally, monocytes that are activated by interferon alpha and/or lipopolysaccharides release exosomes that carry altered miRNA profiles22. These exosomes can alter BMECs and initiate an inflammatory response22. Together, with studies on BBB permeability in mental disorders, the evidence above demonstrates that alterations in BMEC could result in a leaky BBB, leading to an increase in neuroinflammation and onset, or progression of disorders (Fig. 2). Exosome involvement in neuroinflamation has also been documented in mental disorders. EVs isolated from patient serum with ASD stimulated cultured human microglial cells to secrete more pro-inflammatory cytokine interleukin IL-1β81. Another study used an ELISA based method to detect inflammatory markers, in what is suggested to be neural-derived exosomes in a plasma sample. Anti-SNAP25, a neuron marker, was used as the capture antibody, and anti-CD81, a known exosome marker, along with inflammatory markers were used as the detection antibody87. After normalization, the ratio of IL34/CD81 was significantly higher in patients with major depressive disorder (MDD) compared to controls, suggesting increased inflammation87. However, it is important to note that although CD81 is a known exosome marker, it is not exclusive to exosomes and the ELISA based method may be detecting non-EV bound proteins.
Interestingly, central inflammation can be detected systemically via EVs, making them ideal candidates for biomarkers of mental disorders. In one recent study by Couch et al. (2017), brain injury was shown to increase EV release in rats88. EVs from those rats were collected, and injected into healthy rats. The EVs were taken up by the liver where they initiated a systemic acute phase response (APR), a reaction to inflammation for the activation of an early-defense system88. Alterations to the periphery have also been found to affect CNS function as demonstrated by injecting (via tail-vein injection) peripherally-derived exosomes from immune-challenged mice. This led to increased CNS expression of pro-inflammatory cytokine mRNA and associated miRNA in recipient mice20. Given that exosomes can elicit a peripheral response to inflammation88, it would be interesting to investigate whether exosomes may partially explain peripheral changes, such as changes within the gastrointestinal system and gut microbiome observed in mental disorders65.
There is also evidence that exosomes may be a transfer vehicle for translational regulators, specifically via the transfer of miRNA cargo9,83,89. Once exosomes fuse with target cells, they may transfer their miRNA content to recipient cells, where they remain functional9. Sustained changes in gene expression, through epigenetic modifications, are associated with mental disorders14,15,16,17. Expression levels of numerous miRNAs are demonstrably altered in serum17,90 and in postmortem brain tissue of psychiatric patients91,92. The EV cargo, specifically miRNA, could potentially explain in part the modifications in gene expression observed in mental disorders.
Taken together, exosome signalling appears to play a role in gene regulation, plasticity, neurogenesis, and neuroinflammation. Should exosomes mediate such mechanisms in the brain, these nano-vesicles might be critical to further understanding neurobiological changes occurring in mental disorders.
Biomarker potential of exosomes
The ability of exosomes to readily cross the BBB is an important property that renders them as particularly good biomarkers for CNS diseases and treatment response. Of particular interest is the ability to characterize exosomes based on their cell of origin, potentially providing an extra layer of insight into the disease of interest. Currently, much of the cell-specific exosome research is performed in cell culture; however, exosomes from different cell types are diverse, and there has been a recent surge in interest for identifying exosomes of a specific origin from biological fluids93. The potential to access centrally-derived material in the periphery may provide compelling information about the mechanism of a disease, by way of a clinically accessible biomarker.
A mixed population of exosomes (from multiple cell types) can be isolated from biofluids using multiple methods including ultracentrifugation, immunomagnetic beads, and chromatography94,95. Additionally, exosomes have a lipid bilayer; therefore RNAse treatment prior to use will ensure that cargo used downstream was encapsulated within the vesicle96. This mixed population of exosomes may be identified using western blots or mass spectrometry using proteins which are involved in biogenesis of ILVs, including tetraspanins and proteins involved in the ESCRT machinery needed for biogenesis97. It is important to note that many of these markers are not exclusive to exosomes, and further characterizations of exosomes is required.
It is possible to take this one step further and enrich for exosomes derived from a specific cell type from the mixed population of exosomes using cell-specific markers, as exosomes have been found to carry proteins specific to their cell of origin. In psychiatry, investigating cells from the CNS may provide insights toward mechanisms of disease in the brain. Isolating exosomes from those cells that have been implicated in mental disorders may bridge the gap between peripheral biomarkers and mechanistic insight to the disease.
Exosomes released from developing and mature hippocampal neurons contain L1 cell adhesion molecule (L1CAM), and the GluR2/3 subunits of glutamate receptors, both of which are known neuronal markers18,98. Protein markers, such as glial fibrillary acidic protein (GFAP), glutamine aspartate transporter (GLAST), and glutamine synthetase (GLUL), can be used to enrich for astrocytic-derived exosomes11. Additionally, myelin proteolipid protein (PLP) and 2’, 3’-cyclic nucleotide 3’-phosphodiesterase (CNP) have been identified on exosomes derived from oligodendrocytes99. Enriching for a specific cell-derived population of exosomes allows for examination of target cells of interest. In the biomarker field, this may allow for greater connections to form between the marker and mechanisms of disease.
In the last few years, research has been conducted with neuron-derived exosomes to try and answer questions of brain-related disorders from blood biopsies. Sun et al.82 use exosomes isolated from plasma to enrich for neuron-derived exosomes. In doing so, the group identified that both the number of neural-derived exosomes as well as levels of High-mobility group box 1, Neurofilament light, and Amyloid β-proteins may act as potential biomarkers of neuropsychological impairment in HIV82. Neuronal-derived EVs were isolated and concentrations of tau, Aβ42, and IL-10 were elevated in military personal with mild traumatic brain injuries compared to controls100. Neural-derived exosomes from plasma have also been used in a pilot study to investigate protein biomarkers for patients with MDD87. Additionally, other cell-derived exosomes have been studied in the context of other brain-related disorders. Cargo proteins from astrocytic-derived exosomes have been studied for mechanistic insight into Alzheimer’s disease11. The ability to access neural-derived exosomes in plasma shows promising clinical utility in psychiatry.
Future directions
Although the field of exosome investigation remains relatively novel, compelling evidence from other domains indicates that studying exosomes can provide insight into disease mechanisms and processes associated with mental disorders and treatment response. Currently, much of the research on exosomes fixates on biomarkers of disease state, and their ability to mediate cell-to-cell communication. However, more work is needed with respects to mechanisms of bi-directional transfer of exosomes across the BBB. Future studies of exosomes in psychiatry should focus on profiling changes in size or number of exosomes released, and changes in cargo. Additionally, this type of work can be further extended by investigating these differences in a specific cell type. Exosomes derived from cells in the CNS have immense biomarker potential, as they may reflect physiological changes in mental disorders, which can be accessed in the periphery.
References
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am. J. Psychiatry 163, 1905–1917 (2006).
Samanta, S. et al. Exosomes: new molecular targets of diseases. Acta Pharmacol. Sin. 39, 501 (2017).
Lee, Y., El Andaloussi, S. & Wood, M. J. A. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 21(R1), R125–R134 (2012).
Gómez-Molina, C. et al. Small Extracellular Vesicles in Rat Serum ContainAstrocyte-Derived Protein Biomarkers of Repetitive Stress. Int. J. Neuropsychopharmacol. 22, 232–246 (2018).
Gheinani, A. H. et al. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 8, 3945 (2018).
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L. & Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420 (1987).
Gallo, A., Tandon, M., Alevizos, I. & Illei, G. G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE. 7, e30679 (2012).
Guduric-Fuchs, J. et al. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genom. 13, 357 (2012).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Taylor, D. D. & Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21 (2008).
Goetzl, E. J. et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 30, 3853–3859 (2016).
Shi, M. et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 128, 639–650 (2014).
Banigan, M. G. et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE. 8, e48814 (2013).
Baudry, A., Mouillet-Richard, S., Schneider, B., Launay, J.-M. & Kellermann, O. MiR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 329, 1537–1541 (2010).
Beveridge, N. J. et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum. Mol. Genet. 17, 1156–1168 (2008).
Muiños-Gimeno, M. et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol. Psychiatry 69, 526–533 (2011).
Rong, H. et al. MicroRNA-134 plasma levels before and after treatment for bipolar mania. J. Psychiatr. Res. 45, 92–95 (2011).
Lachenal, G. et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 46, 409–418 (2011).
Bahrini, I., Song J-h, Diez, D. & Hanayama, R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci. Rep. 5, 7989 (2015).
Li, J. J. et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflamm. 15, 8 (2018).
Rajendran, L. et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl Acad. Sci. USA 103, 11172–11177 (2006).
Dalvi, P., Sun, B., Tang, N. & Pulliam, L. Immune activated monocyte exosomes alter microRNAs in brain endothelial cells and initiate an inflammatory response through the TLR4/MyD88 pathway. Sci. Rep. 7, 9954 (2017).
Luarte, A. et al. Astrocytes at the hub of the stress response: potential modulation of neurogenesis by miRNAs in astrocyte-derived exosomes. Stem Cells Int. 2017, 1719050. (2017).
Batiz, L. F. et al. Exosomes as novel regulators of adult neurogenic niches. Front. Cell. Neurosci. 9, 501 (2015).
Lafourcade, C., Ramírez, J. P., Luarte, A., Fernández, A. & Wyneken, U. MiRNAs in astrocyte-derived exosomes as possible mediators of neuronal plasticity. J. Exp. Neurosci. 10(Suppl 1), 1–9 (2016).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Mustapic, M. et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front. Neurosci. 11, 278 (2017).
Zhang, G. & Yang, P. A novel cell‐cell communication mechanism in the nervous system: exosomes. J. Neurosci. Res. 96, 45–52 (2018).
Glebov, K. et al. Serotonin stimulates secretion of exosomes from microglia cells. Glia 63, 626–634 (2015).
Altick, A. L., Baryshnikova, L. M., Vu, T. Q. & von Bartheld, C. S. Quantitative analysis of multivesicular bodies (MVBs) in the hypoglossal nerve: Evidence that neurotrophic factors do not use MVBs for retrograde axonal transport. J. Comp. Neurol. 514, 641–657 (2009).
Yohn, C. N., Gergues, M. M. & Samuels, B. A. The role of 5-HT receptors in depression. Molecular. Brain 10, 28 (2017).
Helton, S. G. & Lohoff, F. W. Serotonin pathway polymorphisms and the treatment of major depressive disorder and anxiety disorders. Pharmacogenomics 16, 541–553 (2015).
Yang, A. C. & Tsai, S.-J. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int. J. Mol. Sci. 18, 1689 (2017).
López-Figueroa, A. L. et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol. Psychiatry 55, 225–233 (2004).
Cai, X. et al. Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat. Neurosci. 16, 464 (2013).
Polanco, J. C., Li, C., Durisic, N., Sullivan, R. & Götz, J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol. Commun. 6, 986 (2018).
Chivet, M., Hemming, F., Fraboulet, S. & Sadoul, R. Emerging role of neuronal exosomes in the central nervous system. Front. Physiol 3, 145 (2012).
Frühbeis, C., Fröhlich, D. & Krämer-Albers, E.-M. Emerging roles of exosomes in neuron–glia communication. Front. Physiol. 3, 119 (2012).
Frühbeis, C. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS Biol. 11, e1001604 (2013).
Guitart, K. et al. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 64, 896–910 (2016).
Stephan, K. E., Baldeweg, T. & Friston, K. J. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry 59, 929–939 (2006).
Duman, R. S. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialog. Clin. Neurosci. 11, 239–255 (2009).
Duman, R. S., Aghajanian, G. K., Sanacora, G. & Krystal, J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249 (2016).
Schloesser, R. J., Huang, J., Klein, P. S. & Manji, H. K. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33, 110 (2008).
McKelvey, K. J., Powell, K. L., Ashton, A. W., Morris, J. M. & McCracken, S. A. Exosomes: mechanisms of uptake. J. Circ. Biomark. 4, 7 (2015).
Tian, T., Wang, Y., Wang, H., Zhu, Z. & Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live‐cell microscopy. J. Cell. Biochem. 111, 488–496 (2010).
Mulcahy, L. A., Pink, R. C., Carter, D. R. F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. https://doi.org/10.3402/jev.v3.24641 (2014).
Chivet, M. et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 3, 24722 (2014).
Demory Beckler, M. et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol. Cell. Proteomics 12, 343–355 (2013).
Sinha, M. S. Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta neuropathol. 136, 41–56 (2018).
Kheirandish-Gozal, L., Khalyfa, A. & Gozal, D. Exosomes, blood–brain barrier, and cognitive dysfunction in pediatric sleep apnea. Sleep Biol. Rhythms 15, 261–267 (2017).
Sanchez-Covarrubias, L., Slosky, L. M., Thompson, B. J., Davis, T. P. & Ronaldson, P. T. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr. Pharm. Des. 20, 1422–1449 (2014).
Chen, C. C. et al. Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell. Mol. Bioeng. 9, 509–529 (2016).
Zhuang, X. et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19, 1769–1779 (2011).
Matsumoto, J. et al. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 5, 360 (2017).
Dutta, D. & Donaldson, J. G. Search for inhibitors of endocytosis: intended specificity and unintended consequences. Cell. Logist. 2, 203–208 (2012).
Haraszti, R. A. et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 5, 32570 (2016).
Zhou, W. et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 25, 501–515 (2014).
Paul, D. et al. Appearance of claudin-5+leukocytes in the central nervous system during neuroinflammation: a novel role for endothelial-derived extracellular vesicles. J. Neuroinflamm. 13, 292 (2016).
Nitta, T. et al. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J. Cell. Biol. 161, 653–660 (2003).
Menard, C. et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760 (2017).
Maes, M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro. Endocrinol. Lett. 29, 287–291 (2008).
Najjar, S., Pearlman, D. M., Alper, K., Najjar, A. & Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 10, 43 (2013).
Carabotti, M., Scirocco, A., Maselli, M. A. & Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209 (2015).
Anglin, R., Surette, M., Moayyedi, P. & Bercik, P. Lost in translation: the gut microbiota in psychiatric illness. Can. J. Psychiatry 60, 460–463 (2015).
Fond, G. Inflammation in psychiatric disorders. Eur. Psychiatry 29 (8, Supplement), 551–552 (2014).
Kalimuthu, S. et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front. Pharmacol. 9, 1116 (2018).
Yang, T. et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32, 2003–2014 (2015).
Haney, M. J. et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control Release 207, 18–30 (2015).
Liu, Y. et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 5, 17543 (2015).
Fond, G., Macgregor, A. & Miot, S. Nanopsychiatry—the potential role of nanotechnologies in the future of psychiatry: a systematic review. Eur. Neuropsychopharmacol. 23, 1067–1071 (2013).
Willms, E. et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6, 22519 (2016).
Maas, S. L., De Vrij, J. & Broekman, M. L. Quantification and size-profiling of extracellular vesicles usingtunable resistive pulse sensing. J. Vis. Exp. 92, e51623 (2014).
Van Der Vlist, E. J., Nolte, E. N., Stoorvogel, W., Arkesteijn, G. J. & Wauben, M. H. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat. Protoc. 7, 1311 (2012).
Kabe, Y. et al. Development of a highly sensitive device for counting the number of disease-specific exosomes in human sera. Clin. Chem. 64, 1463–1473 (2018).
van der Pol, E. et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 12, 1182–1192 (2014).
Whiteside, T. L. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 74, 103–141 (2016).
Matsumoto, Y. et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol. Rep. 36, 2535–2543 (2016).
Lea, J. et al. Detection of phosphatidylserine-positive exosomes as a diagnostic marker for ovarian malignancies: a proof of concept study. Oncotarget 8, 14395–14407 (2017).
Gauthier, S. A. et al. Enhanced exosome secretion in Down syndrome brain—a protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol. Commun. 5, 65 (2017).
Tsilioni, I. & Theoharides, T. C. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J. Neuroinflamm. 15, 239 (2018).
Sun, B., Dalvi, P., Abadjian, L., Tang, N. & Pulliam, L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS 31, F9–F17 (2017).
Stoorvogel, W. Functional transfer of microRNA by exosomes. Blood 119, 646–648 (2012).
Zheng, T. et al. Exosomes secreted from HEK293-APP Swe/Ind cells impair the hippocampal neurogenesis. Neurotox. Res. 32, 82–93 (2017).
Goldie, B. J. et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 42, 9195–9208 (2014).
Dozio, V. & Sanchez, J.-C. Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J. Extracell. Vesicles 6, 1302705 (2017).
Kuwano, N. et al. Neuron-related blood inflammatory markers as an objective evaluation tool for major depressive disorder: an exploratory pilot case-control study. J. Affect Disord. 240, 88–98 (2018).
Couch, Y. et al. Circulating endothelial cell-derived extracellular vesicles mediate the acute phase response and sickness behaviour associated with CNS inflammation. Sci. Rep. 7, 9574 (2017).
Di Liegro, C. M., Schiera, G. & di Liegro, I. Extracellular vesicle‐associated RNA as a carrier of epigenetic information. Genes 8, 240 (2017).
Lopez, J. P. et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat. Med. 20, 764–768 (2014).
Smalheiser, N. R. et al. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS ONE. 9, e86469 (2014).
Narahari, A., Hussain, M. & Sreeram, V. MicroRNAs as biomarkers for psychiatric conditions: a review of current research. Innov. Clin. Neurosci. 14, 53–55 (2017).
Zabeo, D. et al. Exosomes purified from a single cell type have diverse morphology. J. Extracell. Vesicles 6, 1329476 (2017).
Yu, L.-L. et al. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed. Res. Int 2018, 1–9 (2018).
Li, P., Kaslan, M., Lee, S. H., Yao, J. & Gao, Z. Progress in exosome isolation techniques. Theranostics 7, 789–804 (2017).
Sharples, R. A., Scicluna, B. J. & Hill, A. F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood AU - Cheng, Lesley. J. Extracell. Vesicles 3, 23743 (2014).
Lötvall, J. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 3, 26913 (2014).
Faure, J. et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 (2006).
Kramer-Albers, E. M. et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteom. Clin. Appl. 1, 1446–1461 (2007).
Gill, J. et al. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 32, 1359–1366 (2018).
Acknowledgements
G.T. holds a Canada Research Chair (Tier 1) and a NARSAD Distinguished Investigator Award. He is supported by grants from the Canadian Institute of Health Research (CIHR) (FDN148374 and EGM141899), and by the Fonds de recherche du Québec - Santé (FRQS) through the Quebec Network on Suicide, Mood Disorders, and Related Disorders. S.S. is funded by the Canadian Institute of Health Research (CIHR). We would like to thank the entire Turecki lab for their feedback and guidance. We would particularly like to thank GF and SST for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saeedi, S., Israel, S., Nagy, C. et al. The emerging role of exosomes in mental disorders. Transl Psychiatry 9, 122 (2019). https://doi.org/10.1038/s41398-019-0459-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-019-0459-9
This article is cited by
-
Multiomics and blood-based biomarkers of electroconvulsive therapy in severe and treatment-resistant depression: study protocol of the DetECT study
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Adipose Tissue Exosome circ_sxc Mediates the Modulatory of Adiposomes on Brain Aging by Inhibiting Brain dme-miR-87-3p
Molecular Neurobiology (2024)
-
Exosome Content–Mediated Signaling Pathways in Multiple Sclerosis
Molecular Neurobiology (2024)
-
A novel isolation method for spontaneously released extracellular vesicles from brain tissue and its implications for stress-driven brain pathology
Cell Communication and Signaling (2023)
-
Use of transcranial low-intensity focused ultrasound for targeted delivery of stem cell-derived exosomes to the brain
Scientific Reports (2023)