Abstract
Although most children with acute lymphoblastic leukemia (ALL) receive fractionated total body irradiation (FTBI) as myeloablative conditioning (MAC) for allogeneic hematopoietic stem cell transplantation (allo-HSCT), it is an important matter of debate if chemotherapy can effectively replace FTBI. To compare outcomes after FTBI versus chemotherapy-based conditioning (CC), we performed a retrospective EBMT registry study. Children aged 2–18 years after MAC for first allo-HSCT of bone marrow (BM) or peripheral blood stem cells (PBSC) from matched-related (MRD) or unrelated donors (UD) in first (CR1) or second remission (CR2) between 2000 and 2012 were included. Propensity score weighting was used to control pretreatment imbalances of the observed variables. 3.054 patients were analyzed. CR1 (1.498): median follow-up (FU) after FTBI (1.285) and CC (213) was 6.8 and 6.1 years. Survivals were not significantly different. CR2 (1.556): median FU after FTBI (1.345) and CC (211) was 6.2 years. Outcomes after FTBI were superior as compared with CC with regard to overall survival (OS), leukemia-free survival (LFS), relapse incidence (RI), and nonrelapse mortality (NRM). However, we must emphasize the preliminary character of the results of this retrospective “real-world-practice” study. These findings will be prospectively assessed in the ALL SCTped 2012 FORUM trial.
Similar content being viewed by others
Introduction
Most children with acute lymphoblastic leukemia (ALL) above 2 years of age being candidate to be treated with allogeneic hematopoietic stem cell transplantation (allo-HSCT) receive myeloablative conditioning (MAC) with a fractionated total body irradiation (FTBI)-containing regimen [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. It is an important matter of debate if chemotherapy can effectively replace FTBI. Due to the known late effects associated with the use of FTBI, which include endocrine complications (growth impairment, hypothyroidism, and delayed onset of puberty), infertility, cognitive impairment, cataracts, and an increased risk for secondary malignancies, avoidance of FTBI in the preparation of allo-HSCT is desirable [18,19,20,21,22,23]. To date, it has not been shown that FTBI can be successfully replaced by chemotherapy during conditioning for pediatric ALL [2, 3, 5, 24,25,26].
To compare outcomes of FTBI versus chemotherapy-based conditioning (CC) in childhood ALL, we performed this international retrospective registry-based study. The study was initiated and conducted on behalf of the Paediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation (EBMT). The primary endpoint was leukemia-free survival (LFS). Overall survival (OS), relapse incidence (RI), nonrelapse mortality (NRM), and incidence of acute graft versus host disease (aGvHD) and chronic GvHD (cGvHD) were the secondary endpoints.
Patients and methods
Children and adolescents aged between 2 and 18 years undergoing a first allo-HSCT for ALL in first (CR1) or second complete remission (CR2) after MAC with either bone marrow (BM) or peripheral blood stem cells (PBSC) from either a matched-related (MRD) or unrelated donor (UD) between 2000 and 2012 were included in the study. This observation period was chosen in order to obtain a reasonable time of follow-up (FU). Moreover, the prospective international randomized ALL SCTped 2012 FORUM trial was started in 2013 and is still recruiting patients. Data were obtained from the EBMT database ProMISe (Project Manager Internet Server) and analyzed in the EBMT study office in Paris, France. The study was performed in accordance with the Declaration of Helsinki. The local institutional review board at each participating site approved the allo-HSCT procedures. Patients and/or their legal guardians gave written informed consent to use clinical data and research participation. All authors had access to the primary clinical data.
Statistical analysis
The study population was divided into two groups (patients in CR1 and CR2). Patients’ demographic and clinical characteristics were summarized using the median and interquartile range for continuous variables and counts and percentages for categorical variables. Preparative regimens were FTBI versus CC. For both remission groups the two conditioning regimens were compared using Fisher’s exact test or χ² test for categorical variables and Wilcoxon rank sum test for continuous variables [27].
Median FU was calculated using the reverse Kaplan–Meier method. The primary endpoint was LFS defined as the probability of being alive and free of disease at any point in time. Thus, death or disease relapse was treated as events. Patients alive and free of disease at their last FU were censored [28, 29]. OS was defined as the probability of survival irrespective of the disease state at any point in time. Patients alive at their last FU were censored. RI was defined as the probability of having experienced a relapse. Death without experiencing a relapse was the competing event. NRM was defined as the probability of dying without previous occurrence of a relapse, which was considered as competing event. Incidences of aGvHD (grade III–IV), cGvHD, and extensive cGvHD were defined as first event of aGvHD (grade III–IV), cGvHD, and extensive cGvHD, respectively. Death and relapse were considered as competing events. OS, RI, NRM, and incidence of acute and cGvHD were secondary endpoints [30].
The inverse probability weighting (IPW) method using the propensity score was used to calculate weights and adjust for confounding factors between the treatment groups [31]. Confounding factors considered age at allo-HSCT, year of allo-HSCT, time from diagnosis to allo-HSCT, cytomegalovirus (CMV) serology, stem cell source, and sex mismatch (female to male versus other).
The weighted Kaplan–Meier method was used to estimate the standardized probability of survival for LFS and OS, and the weighted cumulative incidence function was used to calculate cumulative incidence of relapse (RI), NRM, acute, and cGvHD [27,28,29,30]. P values to evaluate survival differences between the two conditioning regimens were calculated using a weighted proportional hazards Cox model including center as a random effect [32]. Results were expressed as weighted probabilities, weighted cumulative incidences, and hazard ratio with their 95% confidence intervals (95% CI). All tests were two-sided. The type 1 error rate was fixed at 0.05 for determination of factors associated with time to event. Analyses were performed using the R statistical software, Version 3.4.3 (R Development Core Team, Vienna, Austria). Weights were calculated using the twang R package [33]. The date of analysis was October 1, 2018.
Results
Characteristics of study patients
3.054 pediatric patients from European and non-European EBMT centers in 45 countries were included. Between 2000 and 2012, 2.630 patients received a FTBI-based and 424 patients received a chemotherapy-based MAC before allo-HSCT. 1.498 patients (49%) were transplanted in CR1 and 1.556 (51%) in CR2. In the CR1 cohort, median FU was 6.8 years (FTBI group) and 6.1 years (CC group), while in the CR2 cohort, median FU was 6.2 years in the FTBI and in the CC group. In both remission groups, the two conditioning groups differed significantly with regard to age at allo-HSCT, year of allo-HSCT, time from diagnosis to allo-HSCT, stem cell source, and CMV serology (donor/patient, Table 1). These confounding factors and the different sizes of the two conditioning groups requested adjustment by the inverse IPW method (propensity score, see “Statistical analysis”).
Hematopoietic stem cell donors and source
1.626 patients (53%) were grafted from an UD and 1.428 patients (47%) from a MRD. The majority (n = 2.105, 69%) received BM and 949 patients (31%) received PBSC (Table 1).
Preparative regimens
The most commonly applied conditioning regimens were FTBI-based (n = 2.630). In CR1 and CR2, 1.285 (86%) and 1.345 (86%) patients, respectively, received an FTBI-based conditioning. 424 patients received a CC (CR1: n = 213 (14%), CR2 n = 211 (14%), Table 1).
FTBI-based
FTBI/Cy (n = 990, 38%) and FTBI/Eto (n = 784, 30%) were the two most frequent used combinations. The remaining patients received different other FTBI-based combinations (n = 856, 32%, Table 1).
Chemotherapy-based
In the CR1 cohort, 213 patients (14%) received CC. These regimens consisted of Busulfan/Cyclophosphamide (Bu/Cy, n = 68), Bu/Cy/Etoposide (Bu/Cy/Eto, n = 66), Bu/Cytarabine (AraC)/+/−Melphalan (Mel, n = 23), Bu/Cy/Mel (n = 20), Bu/Fludarabine (Flu, n = 20), Bu/Cy/Thiotepa (Thio, n = 14), and Bu/Flu/Thio (n = 2).
In the CR2 cohort, 211 patients (14%) received CC. These regimens consisted of Bu/Cy (n = 68), Bu/Cy/Eto (n = 52), Bu/AraC/+/−Mel (n = 35), Bu/Cy/Thio (n = 18), Bu/Cy/Mel (n = 17), Bu/Flu (n = 13), and Bu/Flu/Thio (n = 8, Table 1).
Outcomes
Patients transplanted in CR1
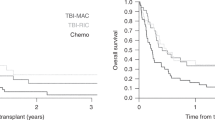
Five years OS was 68.8% (95% CI 66.3–71.5) after FTBI and 74.1% (95% CI 71.1–77.3) after CC (P = 0.25). Five years LFS was 63.8% (95% CI 61.2–66.5) after FTBI and 61.4% (95% CI 58.0–64.9) after CC (P = 0.83). Five years RI was 22.4% (95% CI 20.1–25.0) after FTBI and 26.9% (95% CI 19.7–36.9) after CC (P = 0.33). Five years NRM was 13.8% (95% CI 11.9–15.9) after FTBI and 11.7% (95% CI 6.9–19.8) after CC (P = 0.47). Incidence of aGvHD grade III–IV at day 100 was 11.8% (95% CI 10.1–13.7) after FTBI and 16.9% (95% CI 10.7–26.7) after CC (P = 0.16). Five years incidence of cGvHD was 24.3% (95% CI 21.8–27.1) after FTBI and 20.8% (95% CI 13.7–31.4) after CC (P = 0.60). Five years incidence of extensive cGvHD was 11.3% (95% CI 9.5–13.4) after FTBI and 8.2% (95% CI 4.3–15.9) after CC (P = 0.54, Table 2, Fig. 1a).
Patients transplanted in CR2
FTBI was superior compared with CC in terms of OS, LFS, RI, and NRM. In detail, five years OS was 58.5% (95% CI 56.2–61.6) after FTBI and 35.9% (95% CI 33.0–39.1) after CC (P < 0.0001). Five years LFS was 53.7% (95% CI 51.1–56.5) after FTBI and 29.4% (95% CI 26.6–32.5) after CC (P < 0.0001). Five years RI was 30.6% (95% CI 28.1–33.3) after FTBI and 49.3% (95% CI 40.3–60.2) after CC (P < 0.0001). Five years NRM was 15.7% (95% CI 13.8–17.9) after FTBI and 21.3% (95% CI 15.1–30.2) after CC (P = 0.044).
Significant differences in the incidence of aGvHD grade III–IV at day 100, cGvHD and extensive cGvHD were not detected (Table 2, Fig. 1b).
Discussion
Most pediatric patients with ALL aged above 2 years who undergo allo-HSCT receive FTBI as part of the preparative regimen [1,2,3,4,5, 8,9,10, 12, 13, 15,16,17]. Adverse late effects such as endocrine disorders, infertility, cognitive impairment, cataracts, and increased risk for secondary malignancies, are a major burden of this treatment modality but can at least to a certain extent also occur after CC (e.g., Bu/Cy/Eto) [18,19,20,21,22]. However, to date, it has not been proven whether FTBI could be advantageously omitted from the preparation for allo-HSCT and replaced by CC without jeopardizing LFS [3, 5, 24, 25, 34]. Nevertheless, myeloablative CC remains widely applied in Europe and elsewhere. To compare outcomes of FTBI with CC in pediatric ALL, we performed this multinational retrospective study.
Our study cohort has been intentionally restricted to patients having received first allo-HSCT in CR1 or CR2 after MAC, BM or PBSC as stem cell source from MRD or UD as donors in order to receive a more uniform cohort. In this study, all CC regimens were Bu-based. Bu/Cy, a well-established preparative regimen for pediatric [35,36,37] and adult patients [38, 39], was most frequently applied. Bu/Cy/Eto was the second most frequently used MAC. This combination was applied in the international Berlin–Frankfurt–Münster (iBFM/BFM) clinical trials [1, 40, 41], particularly in infants (Interfant-99) [42, 43], and elsewhere [2, 44,45,46,47]. Within the observation period of this study, the alternative alkylator, treosulfan, was increasingly used for children with malignancies; but no treosulfan-containing regimen reached a significant number of cases [48].
The FTBI and the CC group differed with regard to number of cases, as well as some clinical features, as mentioned above (see Statistical analysis). These potential confounders have been adjusted by the inverse IPW method (propensity score) in order to allow the comparison of the outcomes of the two conditioning groups.
In the CR1 cohort the outcome after FTBI was not significantly different compared with CC. This is a new, interesting finding; although we do not know the reasons for omission of FTBI, which could be manifold: (1) young age, (2) negativity of minimal residual disease before allo-HSCT, (3) high risk for toxicity and infection after having experienced complications during front line therapy, (4) logistical reasons as no access to timely FTBI, and (5) decision of patients/parents. However, due to the large number of participating centers from various countries, there might be some equipoise.
Not surprisingly, overall outcomes of the CR2 cohort were inferior compared with CR1 patients. This was predominantly attributed to a significantly higher RI in the CC group of the CR2 cohort. It was impossible to evaluate risk factors for this difference. One could speculate, that patients with increased risk for toxicity due to pretransplant complications and/or a history of cranial/spinal irradiation were stratified to an irradiation-free conditioning.
Interestingly, outcomes after FTBI were superior as compared with CC with regard to OS, LFS, RI, and NRM in our CR2 cohort. More importantly, superior OS, LFS, and RI of the FTBI cohort did not result in a higher but even a lower NRM compared with the CC cohort.
Various hypotheses concerning the different impact of FTBI on conditioning of ALL patients in CR1 versus CR2 can be made. One could speculate that:
-
(1)
In a retrospective study there is no possibility to identify the background of the decision for the given conditioning. Patients in CR1 with unfavorable prognostic factors might have been conditioned with FTBI and those children with a more favorable risk profile might have been treated with CC. This hypothesis might similarly fit to patients in CR2.
-
(2)
Patients in CR2 had more often extra medullary leukemia and would benefit from FTBI.
-
(3)
Relapsed ALL was more resistant to chemotherapeutic agents and benefited from FTBI as a new treatment element.
The potential superiority of FTBI-based conditioning in pediatric ALL was also demonstrated in literature. In 2000, Davies et al. reported a 3-year LFS of 50% after FTBI versus 35% after Bu-based conditioning (P = 0.005) in a cohort of 627 pediatric patients mainly transplanted in CR1 and CR2 [26]. Three years later, Bunin et al. found a 3-year EFS of 58% after FTBI versus 29% after Bu-based conditioning (P = 0.03) in a randomized cohort of 43 children, transplanted in CR1-3 [2].
The main merit of our study is that it includes a large cohort of pediatric ALL patients who, while in remission, received FTBI as well as myeloablative CC for first allo-HSCT using BM and PBSC from MRD and UD following to several European protocols [39, 40]. Consequently, this retrospective study represents “real-world practice.” On the other hand, this registry-based study has some limitations resulting in the fact that our results must be considered as preliminary. In fact, no data were available on: (1) The administration mode of Bu (intravenous or oral) or use of therapeutic drug monitoring and dose adjustment. (2) Cytogenetics or molecular genetics of ALL. (3) Toxicity or reasons for NRM. (4) Secondary malignancies. (5) Minimal residual disease levels at time of allo-HSCT. (6) Site of relapse after front line ALL therapy or after allo-HSCT. (7) CNS involvement. (8) Date of relapse for patients in CR2. The latter information is necessary for classifying a relapse event as very early, early or late for further patient stratification in classes of risk [49], and for a more detailed analysis of the survival of patients transplanted in CR2. Furthermore, since our retrospective study cohort included B- as well as T-ALL phenotypes, BM and PBSC as stem cell sources, MRD and UD, only children above 2 years of age and spanned an observation time of 13 years, our study population still has a heterogeneous character. Moreover, our non-FTBI-receiving CC cohort is relatively small compared with the FTBI group.
We conclude that FTBI-based conditioning was superior to CC in terms of OS, LFS, RI, and NRM for children undergoing allo-HSCT in CR2, according to the largest study comparing outcomes of FTBI versus CC for first allo-HSCT in pediatric ALL. However, we must stress the preliminary character of the results of this retrospective “real-world-practice” study.
Prospective data comparing FTBI and CC for allo-HSCT in children and adolescents with ALL are urgently needed. Due to the limitations of retrospective studies, it seemed justified to ask whether CC is at least as effective as a FTBI-based conditioning in terms of outcome, toxicity, and late effects in a prospective, preferably randomized clinical trial. The answer to this relevant question will be hopefully obtained by the prospective international, multicenter ALL SCTped 2012 FORUM (“For Omitting Radiation Under Majority Age”) randomized trial, which was initiated in 2012 (EudraCT number: 2012-003032-22).
Change history
19 August 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41409-021-01424-5
References
Peters C, Schrappe M, von Stackelberg A, Schrauder A, Bader P, Ebell W, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors—The ALL-SCT-BFM-2003 Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:1265–74.
Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transpl. 2003;32:543–8.
Marks DI, Forman SJ, Blume KG, Perez WS, Weisdorf DJ, Keating A, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2006;12:438–53.
Litzow MR, Perez WS, Klein JP, Bolwell BJ, Camitta B, Copelan EA, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119:1115–24.
Jamieson CH, Amylon MD, Wong RM, Blume KG. Allogeneic hematopoietic cell transplantation for patients with high-risk acute lymphoblastic leukemia in first or second complete remission using fractionated total-body irradiation and high-dose etoposide: a 15-year experience. Exp Hematol. 2003;31:981–6.
Dopfer R, Henze G, Bender-Gotze C, Ebell W, Ehninger G, Friedrich W, et al. Allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia in second remission after intensive primary and relapse therapy according to the BFM- and CoALL-protocols: results of the German Cooperative Study. Blood. 1991;78:2780–4.
Bader P, Kreyenberg H, Henze GHR, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: The ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–84.
Zhang MJ, Davies SM, Camitta BM, Logan B, Tiedemann K, Eapen M, et al. Comparison of outcomes after HLA-matched sibling and unrelated donor transplantation for children with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18:1204–10.
Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014;123:2017–25.
Hahn T, Wall D, Camitta B, Davies S, Dillon H, Gaynon P, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2005;11:823–61.
Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18:505–22.
Simonin M, Dalissier A, Labopin M, Willasch A, Zecca M, Mouhab A, et al. More chronic GvHD and non-relapse mortality after peripheral blood stem cell compared with bone marrow in hematopoietic transplantation for paediatric acute lymphoblastic leukemia: a retrospective study on behalf of the EBMT Paediatric Diseases Working Party. Bone Marrow Transpl. 2017;52:1071–3.
Tracey J, Zhang MJ, Thiel E, Sobocinski KA, Eapen M. Transplantation conditioning regimens and outcomes after allogeneic hematopoietic cell transplantation in children and adolescents with acute lymphoblastic leukemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2013;19:255–9.
Dalle JH, Balduzzi A, Bader P, Lankester A, Yaniv I, Wachowiak J, et al. Allogeneic stem cell transplantation from HLA-mismatched donors for pediatric patients with acute lymphoblastic leukemia treated according to the 2003 BFM and 2007 international BFM studies: impact of disease risk on outcomes. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24:1848–55.
Wachowiak J, Bettoni C, Lange A, Malicki J, Kaczmarek-Kanold M, Gluszak B, et al. Can busulfan replace fractionated total body irradiation as conditioning regimen for allogeneic bone marrow transplantation in children with acute lymphoblastic leukemia. Acta Haematol Pol. 1995;26:377–84.
Bader P, Salzmann-Manrique E, Balduzzi A, Dalle JH, Woolfrey AE, Bar M, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019;3:3393–405.
Friend BD, Bailey-Olson M, Melton A, Shimano KA, Kharbanda S, Higham C, et al. The impact of total body irradiation-based regimens on outcomes in children and young adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2020;67:e28079.
Socie G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18:348–57.
Baker KS, Bresters D, Sande JE. The burden of cure: long-term side effects following hematopoietic stem cell transplantation (HSCT) in children. Pediatr Clin North Am. 2010;57:323–42.
Bresters D, Emons JA, Nuri N, Ball LM, Kollen WJ, Hannema SE, et al. Ovarian insufficiency and pubertal development after hematopoietic stem cell transplantation in childhood. Pediatr Blood Cancer. 2014;61:2048–53.
Bresters D, Lawitschka A, Cugno C, Potschger U, Dalissier A, Michel G, et al. Incidence and severity of crucial late effects after allogeneic HSCT for malignancy under the age of 3 years: TBI is what really matters. Bone Marrow Transpl. 2016;51:1482–9.
Overbeek A, van den Berg MH, Kremer LC, van den Heuvel-Eibrink MM, Tissing WJ, Loonen JJ, et al. A nationwide study on reproductive function, ovarian reserve, and risk of premature menopause in female survivors of childhood cancer: design and methodological challenges. BMC Cancer. 2012;12:363.
Dalle JH, Lucchini G, Balduzzi A, Ifversen M, Jahnukainen K, Macklon KT, et al. State-of-the-art fertility preservation in children and adolescents undergoing haematopoietic stem cell transplantation: a report on the expert meeting of the Paediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT) in Baden, Austria, 29-30 September 2015. Bone Marrow Transpl. 2017;52:1029–35.
Cross NC, Hughes TP, Feng L, O’Shea P, Bungey J, Marks DI, et al. Minimal residual disease after allogeneic bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: correlations with acute graft-versus-host disease and relapse. Br J Haematol. 1993;84:67–74.
Childhood Acute Lymphoblastic Leukaemia Collaborative Group. Beneficial and harmful effects of anthracyclines in the treatment of childhood acute lymphoblastic leukaemia: a systematic review and meta-analysis. Br J Haematol. 2009;145:376–88.
Davies SM, Ramsay NK, Klein JP, Weisdorf DJ, Bolwell B, Cahn JY, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18:340–7.
Iacobelli S, Committee ES. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2013;48(Suppl 1):S1–37.
Bull K, Spiegelhalter DJ. Survival analysis in observational studies. Stat Med. 1997;16:1041–74.
Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transpl. 2001;28:909–15.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–414.
Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500.
Ridgeway G, McCaffrey DF, Morral AR, Burgette LF, Griffin BA. Toolkit for Weighting and Analysis of Nonequivalent Groups: A Tutorial for the R TWANG Package, Santa Monica, Calif.: RAND Corporation, TL-136/1-NIDA, 2014. As of March 09, 2020: https://www.rand.org/pubs/tools/TL136z1.html.
Pulsipher MA, Wayne AS, Schultz KR. New frontiers in pediatric Allo-SCT: novel approaches for children and adolescents with ALL. Bone Marrow Transpl. 2014;49:1259–65.
Hamidieh A, Kargar M, Jahani M, Alimoghaddam K, Bahar B, Mousavi SA, et al. The outcome of allogeneic hematopoietic stem cell transplants without total body irradiation in pediatric patients with acute lymphoblastic leukemia: single centre experience. J Pediatr Hematol Oncol. 2012;34:101–7.
Shah AJ, Lenarsky C, Kapoor N, Crooks GM, Kohn DB, Parkman R, et al. Busulfan and cyclophosphamide as a conditioning regimen for pediatric acute lymphoblastic leukemia patients undergoing bone marrow transplantation. J Pediatr Hematol Oncol. 2004;26:91–7.
Hamidieh AA, Monzavi SM, Kaboutari M, Behfar M, Esfandbod M. Outcome analysis of pediatric patients with acute lymphoblastic leukemia treated with total body irradiation-free allogeneic hematopoietic stem cell transplantation: comparison of patients with and without central nervous system involvement. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2017;23:2110–7.
Shimoni A, Bielorai B, Toren A, Hardan I, Avigdor A, Yeshurun M, et al. Intravenous busulfan-based conditioning prior to allogeneic hematopoietic stem cell transplantation: myeloablation with reduced toxicity. Exp Hematol. 2003;31:428–34.
Aschan J. Risk assessment in haematopoietic stem cell transplantation: conditioning. Best Pract Res Clin Haematol. 2007;20:295–310.
Peters C, Schrauder A, Schrappe M, von Stackelberg A, Stary J, Yaniv I, et al. Allogeneic haematopoietic stem cell transplantation in children with acute lymphoblastic leukaemia: the BFM/IBFM/EBMT concepts. Bone Marrow Transpl. 2005;35(Suppl 1):S9–11.
Balduzzi A, Dalle JH, Wachowiak J, Yaniv I, Yesilipek A, Sedlacek P, et al. Transplantation in children and adolescents with acute lymphoblastic leukemia from a matched donor versus an HLA-identical sibling: is the outcome comparable? Results from the International BFM ALL SCT 2007 Study. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2019;25:2197–210.
Driessen EM, de Lorenzo P, Campbell M, Felice M, Ferster A, Hann I, et al. Outcome of relapsed infant acute lymphoblastic leukemia treated on the interfant-99 protocol. Leukemia. 2016;30:1184–7.
Mann G, Attarbaschi A, Schrappe M, De Lorenzo P, Peters C, Hann I, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood. 2010;116:2644–50.
von Bueltzingsloewen A, Esperou-Bourdeau H, Souillet G, Demeocq F, Mechinaud-Lacroix F, Michel G, et al. Allogeneic bone marrow transplantation following a busulfan-based conditioning regimen in young children with acute lymphoblastic leukemia: a Cooperative Study of the Societe Francaise de Greffe de Moelle. Bone Marrow Transpl. 1995;16:521–7.
Zander AR, Berger C, Kroger N, Stockshlader M, Kruger W, Horstmann M, et al. High dose chemotherapy with busulfan, cyclophosphamide, and etoposide as conditioning regimen for allogeneic bone marrow transplantation for patients with acute myeloid leukemia in first complete remission. Clin Cancer Res: Off J Am Assoc Cancer Res. 1997;3:2671–5.
Horstmann M, Kroschke G, Stockschlader M, Betker R, Kruger W, Erttmann R, et al. Early toxicity of intensified conditioning with etoposide combined with total body irradiation/cyclophosphamide or busulfan/cyclophosphamide in children undergoing autologous or allogeneic bone marrow transplantation. Pediatr Hematol Oncol. 1996;13:45–53.
Sandler ES, Hagg R, Coppes MJ, Mustafa MM, Gamis A, Kamani N, et al. Hematopoietic stem cell transplantation (HSCT) with a conditioning regimen of busulfan, cyclophosphamide, and etoposide for children with acute myelogenous leukemia (AML): a phase I study of the Pediatric Blood and Marrow Transplant Consortium. Med Pediatr Oncol. 2000;35:403–9.
Boztug H, Sykora KW, Slatter M, Zecca M, Veys P, Lankester A, et al. European society for blood and marrow transplantation analysis of treosulfan conditioning before hematopoietic stem cell transplantation in children and adolescents with hematological malignancies. Pediatr Blood Cancer. 2016;63:139–48.
Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120:2807–16.
Acknowledgements
Presented in parts as oral presentations at the 56th Annual Meeting of the American Society of Hematology (ASH), San Francisco, CA, USA, December 6–9, 2014, at the 59th Annual Meeting of the ASH, Atlanta, GA, USA, December 9–12, 2017, at the 44th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Lisbon, Portugal, March 18–21, 2018, and at the 45th Annual Meeting of the EBMT, Frankfurt/Main, Germany, March 24–27, 2019. Thanks to colleagues who included patients in this study: Alain Fischer, Pediatric Hematology-Immunology and Rheumatology Unit, Assistance Publique-Hôpitaux de Paris, Necker-Enfants Malades University Hospital, Paris, France. Ali Unal, Department of Hematology–Oncology, Erciyes Medical School, Kapadokya, BMT Center, Kayseri, Turkey. Ali Ugur Ural, Department of Hematology, Bayindir Hospital, Ankara, Turkey. Amos Toren, Department of Pediatric Hemato-Oncology, The Cancer Research Center, Sheba Medical Center, Ramat Gan, Israel. Ana Sastre, Servicio de Hemato-Oncología Pediátrica, Hospital Universitario La Paz, Madrid, Spain. Andrew Cant, Department of Pediatric Immunology, Great North Children's Hospital, Newcastle upon Tyne, UK. Andrzej Lange, Lower Silesian Center for Cellular Transplantation with National Bone Marrow Donor Registry, Wroclaw, Poland. Anne Sirvent, Hôpital Arnaud de Villeneuve, CHRU Montpellier, Montpellier, France. Antonia Sampol, Hospital Son Dureta, Palma de Mallorca, Spain. Bernd Gruhn, Department of Pediatrics, Jena University Hospital, Jena, Germany. Brenda Gibson, Royal Hospital for Sick Children, Glasgow, Scotland. Bruno Lioure, Service d’hématologie, Hôpital Hautepierre, Strasbourg, France. Carlos Richard Espiga, Section of Hematology, Hospital U. Marques de Valdecilla, Santander, Spain. Charlotte Jubert, Department of Pediatric Oncology and Hematology, Hôpital Pellegrin, Bordeaux, France. Christian Urban, Children's University Hospital, Medical University of Graz, Graz, Austria. Claudio Favre, Department of Pediatrics, University of Pisa, Pisa, Italy. Cristian Jinca, University of Medicine and Pharmacy „V. Babes”, IIIrd Clinic of Pediatrics, Center for Bone Marrow Transplantation, Timisoara, Romania. David Valcárcel, Hospital Vall D'Hebrón, Barcelona, Spain. Dobrin Konstantinov, Specialized Children’s Oncohaematology Hospital, Sofia, Bulgaria. Dolores Caballero, Hematology Department, University Hospital of Salamanca, Salamanca, Spain. Dragana Stamatovic, Military Medical Academy, Clinic of Hematology, Belgrade, Serbia. Duyu Uçkan-Çetinkaya, Division of Pediatric Hematology, Hacettepe University, Ankara, Turkey. Edoardo Lanino, Institute G. Gaslini, Genova, Italy. Eric Deconinck, Department of Hematology, Jean Minjoz Hospital, Besançon, France. Gaelle Guillerm, Department of Hematology, University Hospital, Brest, France. Gerard Socié, Division of Hematology, Hospital St Louis & University Paris, Paris, France. Giuseppe Bandini, Institute of Haematology, St. Orsola University Hospital, Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy. Giuseppe Milone, Hemopoietic Transplant Program, AOU Policlinico Vittorio Emanuele, Catania, Italy. Giuseppe Visani, Department of Hematology, General Hospital, Pesaro, Italy. Guelsan Sucak, Faculty of Medicine, Department of Haematology, Gazi University, Ankara, Turkey. Gulyuz Ozturk, Division of Pediatric Hematology-Oncology, İstanbul University İstanbul Faculty of Medicine, İstanbul, Turkey. He Huang, Department of Hematology, Nanfang Hospital, Southern Medical University Guangzhou, P. R. China. Henrique Bittencourt, Hematology-Oncology Division, Charles Bruneau Cancer Center, Centre Hospitalier Universitaire Sainte-Justine, Montreal, Quebec, Canada. Herbert Juergens, Children’s University Hospital, Pediatric Hematology and Oncology, Münster, Germany. Ildefonso Espigado, Division of Clinical Hematology, Hospital Universitario Virgen del Rocío, Sevilla, Spain. Inmaculada Heras, Department of Haematology, Hospital Morales Meseguer, Murcia, Spain. Isabel Badell, Hospital Universitari de la Santa Creu i Sant Pau, IIB Sant Pau and Jose Carreras Research Institutes, Barcelona, Spain. Jaques-Olivier Bay, HU Clermont-Ferrand, Service d’Hématologie Clinique Adulte et de Thérapie Cellulaire, Clermont-Ferrand, France. Jerzy Kowalczyk, Department of Paediatric Hematology, Oncology and Transplantology, Medical University, Lublin, Poland. Joan-Hendrik Veelken, Section of Hematology, Leiden University Hospital, Leiden, The Netherlands. Johan Maertens, Department of Hematology, Acute Leukemia and Stem Cell Transplantation Unit, University Hospitals Leuven, Leuven, Belgium. John Snowden, Department of Oncology, University of Sheffield, Sheffield, UK. Jolanta Gozdzik, Department of Clinical Immunology and Transplantation, Polish-American Institute of Pediatrics, Jagiellonian University Medical College, Krakow, Poland. José-Maria Fernandez-Navarro, Pediatric Oncology Unit, Hospital Universitario La Fe, Valencia, Spain. José-Maria Moraleda, Hospital Virgen de la Arrixaca, Murcia, Spain. Josep-Maria Ribera-Santasusana, Department of Clinical Haematology, Catalan Institute of Oncology, Badalona, Spain. Kristina Carlson, Department of Haematology, University Hospital, Uppsala, Sweden. Laurence Clement, Department of Hematology, Nancy CHU, UTMA, Vandoeuvre-lès-Nancy, France. Liisa Volin, Department of Medicine, Helsinki University Central Hospital, Helsinki, Finland. Maija Itälä-Remes, Division of Medicine, Department of Hematology and Stem Cell Transplantation, Turku University Hospital, Turku, Finland. Maria Jesús Pascual-Cascon, UGC de Hematología y Hemoterapia, Hospital Universitario Regional de Málaga, Málaga, Spain. Mario Zecca, Immunology and Transplantation Laboratory/Cell Factory/Paediatric Haematology/Oncology, Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy. Mariusz Wysocki, Department of Pediatric Hematology and Oncology, Collegium Medicum, Nicolaus Copernicus University Torun, Bydgoszcz, Poland. Martin Schrappe, Department of Pediatrics, University Medical Center Schleswig-Holstein, Campus Kiel, Kiel, Germany. Maurizio Musso, Unità Operativa di Oncoematologia e Trapianto di Midollo, Ospedale La Maddalena, Palermo, Italy. Melih Aktan, Division of Hematology, Department of Internal Medicine, Istanbul Medical School, University of Istanbul, Istanbul, Turkey. Michael Albert, University Children’s Hospital, Munich, Germany. Miguel Ángel Sanz, Hematology Department, University Hospital La Fe, Valencia, Spain. Mikhail Maschan, Dmitrii Rogachev Federal Research Center for Pediatric Hematology, Oncology and Immunology, Moscow, Russia. Mouhab Ayas, Department of Pediatric Hematology Oncology, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia. Noel Milpied, Hematology, CHU de Bordeaux, Bordeaux, France. Owen Smith, Pediatric Oncology and Hematology, Our Lady’s Children's Hospital, Crumlin, Dublin, Ireland. Paolo Di Bartolomeo, Department of Hematology, Bone Marrow Transplant Center, Spirito Santo Hospital, Pescara, Italy. Patrice Chevallier, Department D’Hematologie, CHU Nantes, Nantes, France. Pedro Gomez-Garcia, Department of Hematology, Reina Sofia Hospital, Cordoba, Spain. Peter Dreger, Department of Medicine V, University Hospital Heidelberg, Heidelberg, Germany. Renato Fanin, Azienda Ospedaliero Universitaria di Udine, Udine, Italy. Robert Wynn, Blood and Marrow Transplant Unit, Royal Manchester Children’s Hospital, Manchester, UK. Roberto Foá, Division of Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Roma, Italy. Rosanna Scimè, Department of Hematology I, Azienda Ospedali Riuniti Villa Sofia-Cervello, Palermo, Italy. Samo Zver, Department of Haematology, University Medical Centre Ljubljana, Ljubljana, Slovenia. Sandrine Rofine, CHU-Hôpitaux de Rouen, Immuno-Hémato-Oncologie Pediatrique, Hôptial d’enfants, Rouen, France. Sarah Lawson, Bone Marrow Transplant Unit, University Hospital Birmingham NHS Trust, Birmingham, UK. Savas Kansoy, Faculty of Medicine, Pediatric Hematology and Oncology, Ege University, Izmir, Turkey. Thierry Lamy, Department of Hematology, Rennes University Hospital, Rennes, France. Tracey O’Brien, Kids Cancer Centre, Sydney Children’s Hospital, Randwick, NSW, Australia. Ulker Kocak, Faculty of Medicine, Pediatrics, Gazi University, Ankara, Turkey. Victoria Bordon, Department of Pediatric Hemato-Oncology and Stem Cells Transplant, Universitair Ziekenhuis Ghent, Ghent, Belgium. William Arcese, Department of Hematology, Stem Cell Transplant Unit, Rome Transplant Network, “Tor Vergata” University Hospital, Rome, Italy.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Contributions
Conception and design: CP, PB, J-HD, TEK, and AL. Provision of study material or patients: R-MH, VK-R, AY, J-HD, JW, AAH, JS, MB, CD-H, AP, KN, MI, ES, GM, JB, MA, BA, FL, AB, CP, PS, GK, PV, SD, RO, OA, SS, MS, JR, AK, DN, FF, TG, MA, KV, AA, AC, SR, YB, AK, AG, AC, AB, and all colleagues mentioned above. Collection and assembly of data: AD, J-EG, ML. Statistical analysis: J-EG, ML. Data interpretation: AMW, CP, HP, AB, SC, PB. Manuscript writing: AMW, CP, AB, J-EG, PB, JW, PV, J-HD, FL, AL, SC. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Willasch, A.M., Peters, C., Sedláček, P. et al. Myeloablative conditioning for allo-HSCT in pediatric ALL: FTBI or chemotherapy?—A multicenter EBMT-PDWP study. Bone Marrow Transplant 55, 1540–1551 (2020). https://doi.org/10.1038/s41409-020-0854-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0854-0
This article is cited by
-
Evolution and optimization of therapies for acute lymphoblastic leukemia in infants
International Journal of Hematology (2023)
-
Teenagers and young adults with a past of allogenic hematopoietic stem cell transplantation are at significant risk of chronic kidney disease
Pediatric Nephrology (2022)
-
Long-term neurocognitive and quality of life outcomes in survivors of pediatric hematopoietic cell transplant
Journal of Cancer Survivorship (2022)
-
Transplant characteristics and self-reported pulmonary outcomes in Swiss childhood cancer survivors after hematopoietic stem cell transplantation—a cohort study
Bone Marrow Transplantation (2021)
-
The impact of donor type on the outcome of pediatric patients with very high risk acute lymphoblastic leukemia. A study of the ALL SCT 2003 BFM-SG and 2007-BFM-International SG
Bone Marrow Transplantation (2021)