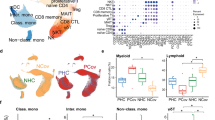
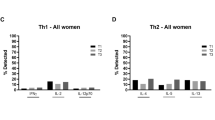
Abstract
Blood CD14+ monocytes are frontline immunomodulators categorized into classical, intermediate or non-classical subsets, and subsequently differentiated into M1 pro- or M2 anti-inflammatory macrophages on stimulation. Although the Zika virus (ZIKV) rapidly establishes viraemia, the target cells and immune responses, particularly during pregnancy, remain elusive. Furthermore, it is unknown whether African- and Asian-lineage ZIKV have different phenotypic impacts on host immune responses. Using human blood infection, we identified CD14+ monocytes as the primary target for African- or Asian-lineage ZIKV infection. When immunoprofiles of human blood infected with ZIKV were compared, a classical/intermediate monocyte-mediated M1-skewed inflammation by the African-lineage ZIKV infection was observed, in contrast to a non-classical monocyte-mediated M2-skewed immunosuppression by the Asian-lineage ZIKV infection. Importantly, infection of the blood of pregnant women revealed an enhanced susceptibility to ZIKV infection. Specifically, Asian-lineage ZIKV infection of pregnant women’s blood led to an exacerbated M2-skewed immunosuppression of non-classical monocytes in conjunction with a global suppression of type I interferon-signalling pathway and an aberrant expression of host genes associated with pregnancy complications. Also, 30 ZIKV+ sera from symptomatic pregnant patients showed elevated levels of M2-skewed immunosuppressive cytokines and pregnancy-complication-associated fibronectin-1. This study demonstrates the differential immunomodulatory responses of blood monocytes, particularly during pregnancy, on infection with different lineages of ZIKV.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Benitez, M. A. Climate change could affect mosquito-borne diseases in Asia. Lancet 373, 1070 (2009).
Chouin-Carneiro, T. et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis. 10, e0004543 (2016).
Zika Situation Report: 20 January 2017 (World Health Organization, 2017).
Enfissi, A., Codrington, J., Roosblad, J., Kazanji, M. & Rousset, D. Zika virus genome from the Americas. Lancet 387, 227–228 (2016).
Wang, L. et al. From mosquitos to humans: genetic evolution of Zika virus. Cell Host Microbe 19, 561–565 (2016).
Simonin, Y. et al. Zika virus strains potentially display different infectious profiles in human neural cells. EBioMedicine 12, 161–169 (2016).
Jurado, K. A. et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight 1, e88461 (2016).
Noronha, L., Zanluca, C., Azevedo, M. L., Luz, K. G. & Santos, C. N. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem. Inst. Oswaldo Cruz 111, 287–293 (2016).
Racicot, K., Kwon, J. Y., Aldo, P., Silasi, M. & Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 72, 107–116 (2014).
Faas, M. M., Spaans, F. & De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 5, 298 (2014).
Brown, M. B., von Chamier, M., Allam, A. B. & Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 5, 606 (2014).
Wong, K. L. et al. The three human monocyte subsets: implications for health and disease. Immunol. Res. 53, 41–57 (2012).
Shechter, R., London, A. & Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat. Rev. Immunol. 13, 206–218 (2013).
Audoy-Rémus, J. et al. Rod-shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J. Neurosci. 28, 10187–10199 (2008).
Kourtis, A. P., Read, J. S. & Jamieson, D. J. Pregnancy and infection. N. Engl. J. Med. 370, 2211–2218 (2014).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Jaguin, M., Houlbert, N., Fardel, O. & Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 281, 51–61 (2013).
Magatti, M. et al. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J. Tissue Eng. Regen. Med. 10, 2193 (2016).
Mack, I. et al. The role of chitin, chitinases, and chitinase-like proteins in pediatric lung diseases. Mol. Cell Pediatr. 2, 3 (2015).
Martines, R. B. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR 65, 159–160 (2016).
Sordet, O. et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood 100, 4446–4453 (2002).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. N. Engl. J. Med. 375, 2321–2334 (2016).
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 375, 2321–2334 (2016).
Ishida, J. et al. Pregnancy-associated homeostasis and dysregulation: lessons from genetically modified animal models. J. Biochem. 150, 5–14 (2011).
Bruchova, H. et al. Effect of maternal tobacco smoke exposure on the placental transcriptome. Placenta 31, 186–191 (2010).
Chaemsaithong, P. et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J. Perinat. Med. 41, 665–681 (2013).
Chen, C. K. et al. Fibronectin levels in normal pregnancy and preeclampsia. J. Formosan Med. Assoc. 93, 921–924 (1994).
Rasanen, J. et al. Maternal serum glycosylated fibronectin as a point-of-care biomarker for assessment of preeclampsia. Am. J. Obstet. Gynecol. 212, 82.e1–82.e9 (2015).
Melo, A. S. et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol. 73, 1407–1416 (2016).
Parra, B. et al. Guillain–Barré syndrome associated with Zika virus infection in Colombia. N. Engl. J. Med 375, 1513–1523 (2016).
Waggoner, J. J. & Pinsky, B. A. Zika virus: diagnostics for an emerging pandemic threat. J. Clin. Microbiol. 54, 860–867 (2016).
Rossi, S. L. et al. Characterization of a novel murine model to study Zika virus. Am. J. Trop. Med. Hyg. 94, 1362–1369 (2016).
Delaloye, J. et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5, e1000480 (2009).
Her, Z. et al. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J. Immunol. 184, 5903–5913 (2010).
Hou, W. et al. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood 119, 3128–3131 (2012).
Osuna, C. E. et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 22, 1448–1455 (2016).
Teng, T. S. et al. Viperin restricts chikungunya virus replication and pathology. J. Clin. Invest. 122, 4447–4460 (2012).
Robinson, T. O., Zhang, M., Ochsenbauer, C., Smythies, L. E. & Cron, R. Q. CD4 regulatory T cells augment HIV-1 expression of polarized M1 and M2 monocyte derived macrophages. Virology 504, 79–87 (2017).
Fischer-Smith, T., Tedaldi, E. M. & Rappaport, J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res. Hum. Retroviruses 24, 417–421 (2008).
Palmer, C. et al. HIV elite controllers have lower frequencies of CD14dimCD16+ inflammatory monocytes with greater CX3CR1-dependent tissue homing potential than viremic controllers (VIR9P. 1138). J. Immunol. 194, 215 (2015).
Zonneveld, R. et al. Three atypical lethal cases associated with acute Zika virus infection in Suriname. IDCases 5, 49–53 (2016).
Halai, U. A. et al. Maternal Zika virus disease severity, virus load, prior dengue antibodies and their relationship to birth outcomes. Clin. Infect. Dis 2017, 472 (2017).
Suy, A. et al. Prolonged Zika virus viremia during pregnancy. N. Engl. J. Med. 375, 2611–2613 (2016).
Driggers, R. W. et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374, 2142–2151 (2016).
Hamel, R. et al. Biology of Zika virus infection in human skin cells. J. Virol. 89, 8880–8896 (2015).
Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091 (2016).
Melgert, B. N. et al. Pregnancy and preeclampsia affect monocyte subsets in humans and rats. PLoS ONE 7, e45229 (2012).
Brooks, D. G. et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12, 1301–1309 (2006).
Brooks, D. G., Lee, A. M., Elsaesser, H., McGavern, D. B. & Oldstone, M. B. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 205, 533–541 (2008).
Redpath, S., Ghazal, P. & Gascoigne, N. R. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9, 86–92 (2001).
Ornelas, A. M. et al. Immune activation in amniotic fluid from Zika virus-associated microcephaly. Ann. Neurol. 81, 152–156 (2017).
Zhu, Z. et al. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg. Microbes Infect. 5, e22 (2016).
Klein, R. S. et al. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79, 11457–11466 (2005).
Bhowmick, S. et al. Induction of IP-10 (CXCL10) in astrocytes following Japanese encephalitis. Neurosci. Lett. 414, 45–50 (2007).
Challis, J. R. et al. Inflammation and pregnancy. Reprod. Sci. 16, 206–215 (2009).
Lanciotti, R. S. et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 14, 1232–1239 (2008).
Acknowledgements
We thank M. Diamond and C. Baronti for providing the ZIKV H/PF/2013 strain, and all the healthy volunteers for blood donations. This work was partly supported by CA200422, CA180779, DE023926, AI073099, AI116585, Hastings Foundation and Fletcher Jones Foundation (J.U.J.), MH106806 (A.B.) and 2T90DE021982-06 (J.W.B.), AI28697 and 1R21AI129534-01 from the National Institute of Allergy and Infectious Diseases/National Institutes of Health (K.N.S.), and CAPES/ 88887.116627/2016-01 from Departamento de Ciência e Tecnologia (DECIT) do Ministério da Saúde do Brasil and Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (P.B.).
Author information
Authors and Affiliations
Contributions
S.-S.F. performed and analysed all the experiments, prepared the figures and wrote the first draft of the manuscript. W.C., Y.C., J.W.B., L.-C.C., Y.C., J.S.Y., J.G., G.C., A.B., K.N.S. and P.B. collaborated for the experimental design and interpretation. S.-S.F. and J.U.J. jointly conceived the experimental design, interpreted the results and wrote subsequent drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figures 1–11, Supplementary Tables 3 and 5, Supplementary References.
Supplementary Data 1
Multiplexed NanoString gene profiling (gene expression fold change relative to mock controls).
Rights and permissions
About this article
Cite this article
Foo, SS., Chen, W., Chan, Y. et al. Asian Zika virus strains target CD14+ blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol 2, 1558–1570 (2017). https://doi.org/10.1038/s41564-017-0016-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0016-3
This article is cited by
-
Shared Molecular Signatures Across Zika Virus Infection and Multiple Sclerosis Highlight AP-1 Transcription Factor as a Potential Player in Post-ZIKV MS-Like Phenotypes
Molecular Neurobiology (2023)
-
The envelope protein of Zika virus interacts with apolipoprotein E early in the infectious cycle and this interaction is conserved on the secreted viral particles
Virology Journal (2022)
-
ZIKV can infect human term placentas in the absence of maternal factors
Communications Biology (2022)
-
An epidemic Zika virus isolate suppresses antiviral immunity by disrupting antigen presentation pathways
Nature Communications (2021)
-
Zika virus NS3 protease induces bone morphogenetic protein-dependent brain calcification in human fetuses
Nature Microbiology (2021)