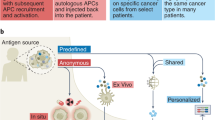
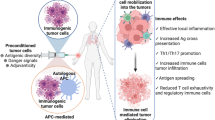
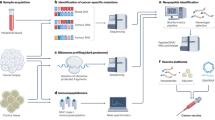
Abstract
Therapeutic cancer vaccines have undergone a resurgence in the past decade. A better understanding of the breadth of tumour-associated antigens, the native immune response and development of novel technologies for antigen delivery has facilitated improved vaccine design. The goal of therapeutic cancer vaccines is to induce tumour regression, eradicate minimal residual disease, establish lasting antitumour memory and avoid non-specific or adverse reactions. However, tumour-induced immunosuppression and immunoresistance pose significant challenges to achieving this goal. In this Review, we deliberate on how to improve and expand the antigen repertoire for vaccines, consider developments in vaccine platforms and explore antigen-agnostic in situ vaccines. Furthermore, we summarize the reasons for failure of cancer vaccines in the past and provide an overview of various mechanisms of resistance posed by the tumour. Finally, we propose strategies for combining suitable vaccine platforms with novel immunomodulatory approaches and standard-of-care treatments for overcoming tumour resistance and enhancing clinical efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wang, S. S. et al. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol. Immunol. 16, 6–18 (2019).
Mollica Poeta, V., Massara, M., Capucetti, A. & Bonecchi, R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front. Immunol. 10, 379 (2019).
Balan, S., Radford, K. J. & Bhardwaj, N. Unexplored horizons of cDC1 in immunity and tolerance. Adv. Immunol. 148, 49–91 (2020).
Fucikova, J. et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 11, 1013 (2020).
Alloatti, A., Kotsias, F., Magalhaes, J. G. & Amigorena, S. Dendritic cell maturation and cross-presentation: timing matters! Immunol. Rev. 272, 97–108 (2016).
Ebrahimi-Nik, H. et al. CD11c+ MHCIIlo GM-CSF-bone marrow-derived dendritic cells act as antigen donor cells and as antigen presenting cells in neoepitope-elicited tumor immunity against a mouse fibrosarcoma. Cancer Immunol. Immunother. 67, 1449–1459 (2018).
Yewdall, A. W., Drutman, S. B., Jinwala, F., Bahjat, K. S. & Bhardwaj, N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS One 5, e11144 (2010).
Ruhland, M. K. et al. Visualizing synaptic transfer of tumor antigens among dendritic cells. Cancer Cell 37, 786–799 e785 (2020). Demonstrates that migratory DCs take up tumour antigens in the TME and transfer these antigen-bearing vesicles to the lymph node-resident DCs for cross-presentation and antitumour T cell activation.
Roberts, E. W. et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016).
Garg, A. D. & Agostinis, P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol. Rev. 280, 126–148 (2017).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Vansteenkiste, J. F. et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 17, 822–835 (2016).
Giaccone, G. et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer 51, 2321–2329 (2015).
Butts, C. et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 59–68 (2014).
Rini, B. I. et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 17, 1599–1611 (2016).
Middleton, G. et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 15, 829–840 (2014).
Lawson, D. H. et al. Randomized, placebo-controlled, phase III Trial of yeast-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) versus peptide vaccination versus GM-CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage IV melanoma: a trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697). J. Clin. Oncol. 33, 4066–4076 (2015).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Prado, F. B. et al. Pharyngeal airway space and frontal and sphenoid sinus changes after maxillomandibular advancement with counterclockwise rotation for class II anterior open bite malocclusions. Dentomaxillofac Radiol. 41, 103–109 (2012).
Atanackovic, D. et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J. Immunol. 172, 3289–3296 (2004).
Ulloa-Montoya, F. et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J. Clin. Oncol. 31, 2388–2395 (2013).
Nemunaitis, J. et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J. Clin. Oncol. 24, 4721–4730 (2006).
Butts, C. et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J. Clin. Oncol. 23, 6674–6681 (2005).
Kirkwood, J. M. et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group phase II trial E1696. Clin. Cancer Res. 15, 1443–1451 (2009).
van Esch, E. M. et al. Expression of coinhibitory receptors on T cells in the microenvironment of usual vulvar intraepithelial neoplasia is related to proinflammatory effector T cells and an increased recurrence-free survival. Int. J. Cancer 136, E95–E106 (2015).
Abdulrahman, Z. et al. A pre-existing coordinated inflammatory microenvironment is associated with complete response of vulvar high-grade squamous intraepithelial lesions to different forms of immunotherapy. Int. J. Cancer 15, 2914-2923.
Abdulrahman, Z. et al. Pre-existing inflammatory immune microenvironment predicts the clinical response of vulvar high-grade squamous intraepithelial lesions to therapeutic HPV16 vaccination. J. Immunother. Cancer 8, e000563 (2020). Shows that clinical responses with vaccines are obtained only in ‘hot’ tumours and that vaccination cannot overcome a cold tumour microenvironment.
Melief, C. J. M. et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci. Transl. Med. 12, eaaz8235 (2020). First to show in patients that vaccine efficacy can be boosted by alleviating local and systemic immunosuppression by MDSCs, resulting in a stronger T cell response and clinical benefit.
Hazama, S. et al. A phase IotaI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J. Transl. Med. 12, 108 (2014).
van Poelgeest, M. I. et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 11, 88 (2013).
Hansen, G. L., Gaudernack, G., Brunsvig, P. F., Cvancarova, M. & Kyte, J. A. Immunological factors influencing clinical outcome in lung cancer patients after telomerase peptide vaccination. Cancer Immunol. Immunother. 64, 1609–1621 (2015).
Talebian Yazdi, M. et al. Local and systemic XAGE-1b-specific immunity in patients with lung adenocarcinoma. Cancer Immunol. Immunother. 64, 1109–1121 (2015).
Santegoets, S. et al. The blood mMDSC to DC ratio is a sensitive and easy to assess independent predictive factor for epithelial ovarian cancer survival. Oncoimmunology 7, e1465166 (2018).
Bailur, J. K., Gueckel, B., Derhovanessian, E. & Pawelec, G. Presence of circulating Her2-reactive CD8+ T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. 17, 34 (2015).
Weide, B. et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin. Cancer Res. 20, 1601–1609 (2014).
Welters, M. J. et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc. Natl Acad. Sci. USA 107, 11895–11899 (2010).
Scurr, M. et al. Effect of modified vaccinia Ankara-5T4 and low-dose cyclophosphamide on antitumor immunity in metastatic colorectal cancer: a randomized clinical trial. JAMA Oncol. 3, e172579 (2017).
Hipp, M. M. et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 111, 5610–5620 (2008).
Rettig, L. et al. Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int. J. Cancer 129, 832–838 (2011).
Suzuki, E., Kapoor, V., Jassar, A. S., Kaiser, L. R. & Albelda, S. M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005).
Vermeij, R. et al. Potentiation of a p53-SLP vaccine by cyclophosphamide in ovarian cancer: a single-arm phase II study. Int. J. Cancer 131, E670–E680 (2012).
Borch, T. H. et al. mRNA-transfected dendritic cell vaccine in combination with metronomic cyclophosphamide as treatment for patients with advanced malignant melanoma. Oncoimmunology 5, e1207842 (2016).
Camisaschi, C. et al. Effects of cyclophosphamide and IL-2 on regulatory CD4+ T cell frequency and function in melanoma patients vaccinated with HLA-class I peptides: impact on the antigen-specific T cell response. Cancer Immunol. Immunother. 62, 897–908 (2013).
Dijkgraaf, E. M. et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum-resistant ovarian cancer. Oncotarget 6, 32228–32243 (2015).
Finke, J. H. et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin. Cancer Res. 14, 6674–6682 (2008).
van Dongen, M. et al. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int. J. Cancer 127, 899–909 (2010).
Sevko, A. et al. Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J. Invest. Dermatol. 133, 1610–1619 (2013).
Dehlin, M., Andersson, S., Erlandsson, M., Brisslert, M. & Bokarewa, M. Inhibition of fms-like tyrosine kinase 3 alleviates experimental arthritis by reducing formation of dendritic cells and antigen presentation. J. Leukoc. Biol. 90, 811–817 (2011).
van der Burg, S. H. Correlates of immune and clinical activity of novel cancer vaccines. Semin. Immunol. 39, 119–136 (2018).
Bocchia, M. et al. Complete molecular response in CML after p210 BCR-ABL1-derived peptide vaccination. Nat. Rev. Clin. Oncol. 7, 600–603 (2010).
Suzuki, S. et al. Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology 5, e1238542 (2016).
Ishikawa, H. et al. Phase I clinical trial of vaccination with LY6K-derived peptide in patients with advanced gastric cancer. Gastric Cancer 17, 173–180 (2014).
Yoshitake, Y. et al. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin. Cancer Res. 21, 312–321 (2015).
Hazama, S. et al. A phase I study of combination vaccine treatment of five therapeutic epitope-peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J. Transl. Med. 12, 63 (2014).
Asahara, S., Takeda, K., Yamao, K., Maguchi, H. & Yamaue, H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J. Transl. Med. 11, 291 (2013).
Koster, B. D. et al. Autologous tumor cell vaccination combined with systemic CpG-B and IFN-alpha promotes immune activation and induces clinical responses in patients with metastatic renal cell carcinoma: a phase II trial. Cancer Immunol. Immunother. 68, 1025–1035 (2019).
Kotsakis, A. et al. A phase II trial evaluating the clinical and immunologic response of HLA-A2+ non-small cell lung cancer patients vaccinated with an hTERT cryptic peptide. Lung Cancer 86, 59–66 (2014).
Iclozan, C., Antonia, S., Chiappori, A., Chen, D. T. & Gabrilovich, D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol. Immunother. 62, 909–918 (2013).
Chiang, C. L. et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin. Cancer Res. 19, 4801–4815 (2013).
Wen, P. Y. et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin. Cancer Res. 25, 5799–5807 (2019).
Trimble, C. L. et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386, 2078–2088 (2015).
Choi, Y. J. et al. A phase II, prospective, randomized, multicenter, open-label study of GX-188E, an HPV DNA vaccine, in patients with cervical intraepithelial neoplasia 3. Clin. Cancer Res. 26, 1616–1623 (2020).
Kim, T. J. et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 5, 5317 (2014).
Kenter, G. G. et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 361, 1838–1847 (2009). First demonstration of clinical efficacy for SLP vaccines.
van Poelgeest, M. I. et al. Vaccination against oncoproteins of HPV16 for noninvasive vulvar/vaginal lesions: lesion clearance is related to the strength of the T-cell response. Clin. Cancer Res. 22, 2342–2350 (2016). Confirmation of clinical efficacy for SLP vaccines in the second independent study.
Massarelli, E. et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 5, 67–73 (2019). First evidence that therapeutic vaccination combined with anti-PD1 leads to a better clinical outcome (doubling of overall response rate and of overall survival).
Aggarwal, C. et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin. Cancer Res. 25, 110–124 (2019). Describes one patient who after therapeutic vaccination and clinical progression was treated with anti-PD1 and had a complete response, thus constituting an anecdotal clinical response in a single patient.
Oh, J. et al. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol. Oncol. 143, 504–510 (2016).
Yoshimura, K. et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur. Urol. 70, 35–41 (2016).
Noguchi, M. et al. an open-label, randomized phase ii trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin. Cancer Res. 22, 54–60 (2016).
Mittendorf, E. A. et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann. Oncol. 27, 1241–1248 (2016).
Pavlick, A. et al. Combined vaccination with NY-ESO-1 protein, poly-ICLC, and Montanide improves humoral and cellular immune responses in patients with high-risk melanoma. Cancer Immunol. Res. 8, 70–80 (2020).
Comiskey, M. C., Dallos, M. C. & Drake, C. G. Immunotherapy in prostate cancer: teaching an old dog new tricks. Curr. Oncol. Rep. 20, 75 (2018).
van der Burg, S. H., Arens, R., Ossendorp, F., van Hall, T. & Melief, C. J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 16, 219–233 (2016).
Melief, C. J., van Hall, T., Arens, R., Ossendorp, F. & van der Burg, S. H. Therapeutic cancer vaccines. J. Clin. Invest. 125, 3401–3412 (2015).
Tomaic, V. Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers (Basel) 8, 95 (2016).
Smith, C. C. et al. Alternative tumour-specific antigens. Nat. Rev. Cancer 19, 465–478 (2019).
Roudko, V. et al. Shared immunogenic poly-epitope frameshift mutations in microsatellite unstable tumors. Cell 183, 1634–1649 e1617 (2020). Identifies highly immunogenic shared polyepitope neoantigens that could generate unique neoantigens after vaccination based on patient-specific HLA expression.
Engelhard, V. H. et al. MHC-restricted phosphopeptide antigens: preclinical validation and first-in-humans clinical trial in participants with high-risk melanoma. J Immunother Cancer 8, e000262 (2020).
Brentville, V. A., Vankemmelbeke, M., Metheringham, R. L. & Durrant, L. G. Post-translational modifications such as citrullination are excellent targets for cancer therapy. Semin. Immunol. 47, 101393 (2020).
Malaker, S. A. et al. Identification of glycopeptides as posttranslationally modified neoantigens in leukemia. Cancer Immunol. Res. 5, 376–384 (2017).
Yarchoan, M. et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 4, e126908 (2019).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Liu, D. et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat. Med. 25, 1916–1927 (2019).
Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 6, 157 (2018).
Miao, D. et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359, 801–806 (2018).
Roudko, V., Greenbaum, B. & Bhardwaj, N. Computational prediction and validation of tumor-associated neoantigens. Front. Immunol. 11, 27 (2020).
Verdegaal, E. M. et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature 536, 91–95 (2016).
Linnemann, C. et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 21, 81–85 (2015).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Matsushita, H. et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (2017).
Chowell, D. et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359, 582–587 (2018).
Lazaro, A. et al. Human leukocyte antigen (HLA) typing by DNA sequencing. Methods Mol. Biol. 1034, 161–195 (2013).
Alioto, T. S. et al. A comprehensive assessment of somatic mutation detection in cancer using whole-genome sequencing. Nat. Commun. 6, 10001 (2015).
Wang, Q., Shashikant, C. S., Jensen, M., Altman, N. S. & Girirajan, S. Novel metrics to measure coverage in whole exome sequencing datasets reveal local and global non-uniformity. Sci. Rep. 7, 885 (2017).
Jennings, L. J. et al. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 19, 341–365 (2017).
Carreno, B. M. et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348, 803–808 (2015).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Alspach, E. et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701 (2019). Important study that shows the role of CD4+ T cells in the TME.
Ott, P. A. et al. A Phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell 183, 347–362 e324 (2020). Important study that shows the role of CD4+ T cells in the TME and the need for tumour specificity as they need to be reactivated also locally in the tumour.
van den Bulk, J. et al. Neoantigen-specific immunity in low mutation burden colorectal cancers of the consensus molecular subtype 4. Genome Med. 11, 87 (2019).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020). Demonstrates that a strong delivery platform, the intravenously administered nanoparticulate liposomal RNA vaccine, can overcome central tolerance to self non-mutated tumour-associated antigens and yield a clinical and immunological response.
Hilf, N. et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565, 240–245 (2019).
Frank, M. J. et al. Autologous tumor cell vaccine induces antitumor T cell immune responses in patients with mantle cell lymphoma: a phase I/II trial. J Exp Med 217, e20191712 (2020).
Liau, L. M. et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 16, 142 (2018).
Maeng, H., Terabe, M. & Berzofsky, J. A. Cancer vaccines: translation from mice to human clinical trials. Curr. Opin. Immunol. 51, 111–122 (2018).
Vormehr, M., Tureci, O. & Sahin, U. Harnessing tumor mutations for truly individualized cancer vaccines. Annu. Rev. Med. 70, 395–407 (2019).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Rosalia, R. A. et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur. J. Immunol. 43, 2554–2565 (2013).
Duperret, E. K. et al. A synthetic DNA, multi-neoantigen vaccine drives predominately MHC class I CD8+ T-cell responses, impacting tumor challenge. Cancer Immunol. Res. 7, 174–182 (2019).
Oynebraten, I., Hinkula, J., Fredriksen, A. B. & Bogen, B. Increased generation of HIV-1 gp120-reactive CD8+ T cells by a DNA vaccine construct encoding the chemokine CCL3. PLoS One 9, e104814 (2014).
Lysen, A. et al. Dendritic cell targeted Ccl3- and Xcl1-fusion DNA vaccines differ in induced immune responses and optimal delivery site. Sci. Rep. 9, 1820 (2019).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Heidegger, S. et al. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with immune checkpoint blockade. EBioMedicine 41, 146–155 (2019).
Reinhard, K. et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020).
Feltkamp, M. C. et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur. J. Immunol. 23, 2242–2249 (1993).
Speiser, D. E. et al. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc. Natl Acad. Sci. USA 105, 3849–3854 (2008).
Appay, V. et al. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J. Immunol. 177, 1670–1678 (2006).
Speiser, D. E. et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J. Clin. Invest. 115, 739–746 (2005).
Zwaveling, S. et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J. Immunol. 169, 350–358 (2002).
Bijker, M. S. et al. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur. J. Immunol. 38, 1033–1042 (2008).
Bijker, M. S. et al. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J. Immunol. 179, 5033–5040 (2007).
Borst, J., Ahrends, T., Babala, N., Melief, C. J. M. & Kastenmuller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647 (2018).
Hailemichael, Y. et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 19, 465–472 (2013).
Welters, M. J. et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 8, 334ra352 (2016).
Sabbatini, P. et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin. Cancer Res. 18, 6497–6508 (2012).
Baumgaertner, P. et al. Vaccination of stage III/IV melanoma patients with long NY-ESO-1 peptide and CpG-B elicits robust CD8+ and CD4+ T-cell responses with multiple specificities including a novel DR7-restricted epitope. Oncoimmunology 5, e1216290 (2016).
Melssen, M. M. et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. J. Immunother. Cancer 7, 163 (2019).
Ossendorp, F., Mengede, E., Camps, M., Filius, R. & Melief, C. J. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J. Exp. Med. 187, 693–702 (1998).
Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R. & Melief, C. J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393, 480–483 (1998).
Avigan, D., Rosenblatt, J. & Kufe, D. Dendritic/tumor fusion cells as cancer vaccines. Semin. Oncol. 39, 287–295 (2012).
Sundarasetty, B. S. et al. Lentivirus-induced ‘Smart’ dendritic cells: pharmacodynamics and GMP-compliant production for immunotherapy against TRP2-positive melanoma. Gene Ther. 22, 707–720 (2015).
Wilgenhof, S. et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol. 34, 1330–1338 (2016).
Gross, S. et al. Twelve-year survival and immune correlates in dendritic cell-vaccinated melanoma patients. JCI Insight 2, e91438 (2017).
Le Gall, C. M., Weiden, J., Eggermont, L. J. & Figdor, C. G. Dendritic cells in cancer immunotherapy. Nat. Mater. 17, 474–475 (2018).
Westdorp, H. et al. Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J. Immunother. Cancer 7, 302 (2019).
Sprooten, J. et al. Trial watch: dendritic cell vaccination for cancer immunotherapy. Oncoimmunology 8, e1638212 (2019).
Bourdely, P. et al. Transcriptional and functional analysis of CD1c+ human dendritic cells identifies a CD163+ subset priming CD8+CD103+ T cells. Immunity 53, 335–352 e338 (2020).
Saxena, M. & Bhardwaj, N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer 4, 119–137 (2018).
Villani, A. C. et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017). Performs unbiased single-cell sequencing and identifies novel DC subsets in the blood.
Balan, S. et al. Large-scale human dendritic cell differentiation revealing Notch-dependent lineage bifurcation and heterogeneity. Cell Rep. 24, 1902–1915 e1906 (2018). Develops a culture system to differentiate and expand, to a large scale, different DC subsets from stem cells.
Moynihan, K. D. et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016).
van der Maaden, K. et al. Hollow microneedle-mediated micro-injections of a liposomal HPV E743-63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Rel. 269, 347–354 (2018).
Lynn, G. M. et al. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol. 38, 320–332 (2020).
Baharom, F. et al. Intravenous nanoparticle vaccination generates stem-like TCF1+ neoantigen-specific CD8+ T cells. Nat. Immunol. 22, 41–52 (2021). Demonstrates that the vaccination route can affect the quality of the immune response elicited.
Li, A. W. et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 17, 528–534 (2018).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Hammerich, L., Bhardwaj, N., Kohrt, H. E. & Brody, J. D. In situ vaccination for the treatment of cancer. Immunotherapy 8, 315–330 (2016).
Bhatia, S. et al. Intratumoral G100, a TLR4 agonist, induces antitumor immune responses and tumor regression in patients with Merkel cell carcinoma. Clin. Cancer Res. 25, 1185–1195 (2019).
Frank, M. J. et al. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 8, 1258–1269 (2018).
Kyi, C. et al. Therapeutic immune modulation against solid cancers with intratumoral poly-ICLC: a pilot trial. Clin. Cancer Res. 24, 4937–4948 (2018).
Ribas, A. et al. SD-101 in combination with pembrolizumab in advanced melanoma: results of a phase Ib, multicenter study. Cancer Discov. 8, 1250–1257 (2018).
Salazar, A. M., Erlich, R. B., Mark, A., Bhardwaj, N. & Herberman, R. B. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol. Res. 2, 720–724 (2014).
Gravekamp, C. & Chandra, D. Targeting STING pathways for the treatment of cancer. Oncoimmunology 4, e988463 (2015).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Ramanjulu, J. M. et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 564, 439–443 (2018).
Redelman-Sidi, G., Glickman, M. S. & Bochner, B. H. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat. Rev. Urol. 11, 153–162 (2014).
Kamath, P., Darwin, E., Arora, H. & Nouri, K. A review on imiquimod therapy and discussion on optimal management of basal cell carcinomas. Clin. Drug. Investig. 38, 883–899 (2018).
Tio, D. et al. A systematic review on the role of imiquimod in lentigo maligna and lentigo maligna melanoma: need for standardization of treatment schedule and outcome measures. J. Eur. Acad. Dermatol. Venereol. 31, 616–624 (2017).
Salmon, H. et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016).
Barry, K. C. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24, 1178–1191 (2018). Defines a novel axis where FLT3L derived from NK cells in the TME induces intratumoural DC recruitment.
Beatty, G. L., Li, Y. & Long, K. B. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert. Rev. Anticancer. Ther. 17, 175–186 (2017).
Piechutta, M. & Berghoff, A. S. New emerging targets in cancer immunotherapy: the role of cluster of differentiation 40 (CD40/TNFR5). ESMO Open 4, e000510 (2019).
Hammerich, L. et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 25, 814–824 (2019).
O’Hara, M. H. et al. Abstract CT004: a phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. https://doi.org/10.1158/1538-7445.AM2019-CT004 (2019).
Bhardwaj, N. et al. Flt3 ligand augments immune responses to anti-DEC-205-NY-ESO-1 vaccine through expansion of dendritic cell subsets. Nature Cancer, https://doi.org/10.1038/s43018-020-00143-y (2020). Demonstrates evidence in a randomized human trial that combination with agents that mobilize and expand the DC population, such as FLT3L, improve a vaccine’s potential for inducing potent and durable antitumour immunity.
Harrington, K., Freeman, D. J., Kelly, B., Harper, J. & Soria, J. C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug. Discov. 18, 689–706 (2019).
Wang, G. et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 11, 1395 (2020).
Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015).
Saxena, M. & Bhardwaj, N. Turbocharging vaccines: emerging adjuvants for dendritic cell based therapeutic cancer vaccines. Curr. Opin. Immunol. 47, 35–43 (2017).
Demaria, S., Coleman, C. N. & Formenti, S. C. Radiotherapy: changing the game in immunotherapy. Trends Cancer 2, 286–294 (2016).
Pilones, K. A. et al. Radiotherapy cooperates with IL15 to induce antitumor immune responses. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.CIR-19-0338 (2020).
Garris, C. S. et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity 49, 1148–1161 e1147 (2018).
Sharma, M. et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat. Commun. 11, 661 (2020).
Diab, A. et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov. https://doi.org/10.1158/2159-8290.CD-19-1510 (2020).
Lopes, J. E. et al. ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J. Immunother. Cancer 8, e000673 (2020).
Weide, B. et al. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol. Immunother. 60, 487–493 (2011).
Bhatia, S. et al. Intratumoral delivery of plasmid IL12 via electroporation leads to regression of injected and noninjected tumors in Merkel cell carcinoma. Clin. Cancer Res. 26, 598–607 (2020).
Greaney, S. K. et al. Intratumoral PLASMID IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral t-cell responses. Cancer Immunol. Res. 8, 246–254 (2020).
Hill, H. C. et al. Cancer immunotherapy with interleukin 12 and granulocyte-macrophage colony-stimulating factor-encapsulated microspheres: coinduction of innate and adaptive antitumor immunity and cure of disseminated disease. Cancer Res. 62, 7254–7263 (2002).
Barrett, J. A. et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 25, 106–116 (2018).
Hewitt, S. L. et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res. 26, 6284–6298 (2020).
Kalbasi, A. & Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020).
Wang, Z. & Cao, Y. J. Adoptive cell therapy targeting neoantigens: a frontier for cancer research. Front. Immunol. 11, 176 (2020).
Miyauchi, S. et al. HPV16 E5 mediates resistance to PD-L1 blockade and can be targeted with rimantadine in head and neck cancer. Cancer Res. 80, 732–746 (2020).
Gurjao, C. et al. Intrinsic resistance to immune checkpoint blockade in a mismatch repair-deficient colorectal cancer. Cancer Immunol. Res. 7, 1230–1236 (2019).
Chew, G. L. et al. DUX4 suppresses MHC class I to promote cancer immune evasion and resistance to checkpoint blockade. Dev. Cell 50, 658–671 e657 (2019).
Huang, L. et al. The RNA-binding protein MEX3B mediates resistance to cancer immunotherapy by downregulating HLA-A expression. Clin. Cancer Res. 24, 3366–3376 (2018).
Kriegsman, B. A. et al. Frequent loss of IRF2 in cancers leads to immune evasion through decreased MHC class I antigen presentation and increased PD-L1 expression. J. Immunol. 203, 1999–2010 (2019).
Ramakrishnan, R. et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J. Immunol. 192, 2920–2931 (2014).
Song, M. et al. IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 562, 423–428 (2018).
Gettinger, S. et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 7, 1420–1435 (2017).
Tran, E. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 375, 2255–2262 (2016).
Cabrera, T. et al. HLA class I expression in metastatic melanoma correlates with tumor development during autologous vaccination. Cancer Immunol. Immunother. 56, 709–717 (2007).
Carretero, R. et al. Bacillus Calmette-Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int. J. Cancer 129, 839–846 (2011).
Paulson, K. G. et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat. Commun. 9, 3868 (2018).
Shin, D. S. et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 7, 188–201 (2017).
Kearney, C. J. et al. Tumor immune evasion arises through loss of TNF sensitivity. Sci Immunol 3, eaar3451 (2018).
Gao, J. et al. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404 e399 (2016).
Gooden, M. et al. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc. Natl Acad. Sci. USA 108, 10656–10661 (2011).
Talebian Yazdi, M. et al. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget 7, 3477–3488 (2016).
Manguso, R. T. et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 547, 413–418 (2017).
van Montfoort, N. et al. NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell 175, 1744–1755 e1715 (2018).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Peng, W. et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Luke, J. J., Bao, R., Sweis, R. F., Spranger, S. & Gajewski, T. F. WNT/beta-catenin pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 25, 3074–3083 (2019).
Trujillo, J. A. et al. Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer 7, 295 (2019).
Morisaki, T. et al. Intranodal administration of neoantigen peptide-loaded dendritic cell vaccine elicits epitope-specific T cell responses and clinical effects in a patient with chemorefractory ovarian cancer with malignant ascites. Immunol Invest, 1–18 (2020).
Chao, M. P., Majeti, R. & Weissman, I. L. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer 12, 58–67 (2011).
Zer, A. et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin. Lung Cancer 19, 426–434 e421 (2018).
Capone, M. et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 6, 74 (2018).
Mariathasan, S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Mazzarella, L. et al. The evolving landscape of ‘next-generation’ immune checkpoint inhibitors: A review. Eur. J. Cancer 117, 14–31 (2019).
Weinberg, F., Ramnath, N. & Nagrath, D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers (Basel) 11, 1191 (2019).
Brown, J. M., Recht, L. & Strober, S. The promise of targeting macrophages in cancer therapy. Clin. Cancer Res. 23, 3241–3250 (2017).
Singel, K. L. et al. Mature neutrophils suppress T cell immunity in ovarian cancer microenvironment. JCI Insight 4, e122311 (2019).
Liu, Y. et al. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol. Immunother. 58, 687–697 (2009).
Ruffell, B. & Coussens, L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–472 (2015).
Saleh, R. & Elkord, E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. 457, 168–179 (2019).
Lin, Y. et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia 21, 1133–1142 (2019).
Flavell, R. A., Sanjabi, S., Wrzesinski, S. H. & Licona-Limon, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 10, 554–567 (2010).
Chuang, Y., Hung, M. E., Cangelose, B. K. & Leonard, J. N. Regulation of the IL-10-driven macrophage phenotype under incoherent stimuli. Innate Immun. 22, 647–657 (2016).
Granot, Z. & Jablonska, J. Distinct functions of neutrophil in cancer and its regulation. Mediators Inflamm. 2015, 701067 (2015).
Tormoen, G. W., Crittenden, M. R. & Gough, M. J. Role of the immunosuppressive microenvironment in immunotherapy. Adv. Radiat. Oncol. 3, 520–526 (2018).
Fu, C. & Jiang, A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front. Immunol. 9, 3059 (2018).
Oweida, A. et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin. Cancer Res. 24, 5368–5380 (2018).
Limagne, E. et al. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. Oncoimmunology 8, e1564505 (2019).
van Deventer, H. W. et al. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 70, 10161–10169 (2010).
Tsuji, K. et al. Induction of immune response against NY-ESO-1 by CHP-NY-ESO-1 vaccination and immune regulation in a melanoma patient. Cancer Immunol. Immunother. 57, 1429–1437 (2008).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Demircioglu, F. et al. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat. Commun. 11, 1290 (2020).
Lakins, M. A., Ghorani, E., Munir, H., Martins, C. P. & Shields, J. D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T cells to protect tumour cells. Nat. Commun. 9, 948 (2018).
Chakravarthy, A., Khan, L., Bensler, N. P., Bose, P. & De Carvalho, D. D. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9, 4692 (2018).
Harryvan, T. J., Verdegaal, E. M. E., Hardwick, J. C. H., Hawinkels, L. & van der Burg, S. H. Targeting of the cancer-associated fibroblast-T-cell axis in solid malignancies. J. Clin. Med. 8, 1989 (2019).
Oshi, M. et al. M1 macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 10, 16554 (2020).
Pathria, P., Louis, T. L. & Varner, J. A. Targeting tumor-associated macrophages in cancer. Trends Immunol. 40, 310–327 (2019).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Thoreau, M. et al. Vaccine-induced tumor regression requires a dynamic cooperation between T cells and myeloid cells at the tumor site. Oncotarget 6, 27832–27846 (2015).
van der Sluis, T. C. et al. Therapeutic peptide vaccine-induced CD8 T cells strongly modulate intratumoral macrophages required for tumor regression. Cancer Immunol. Res. 3, 1042–1051 (2015). Van der Sluis et al. (2015) and van Elsas et al. (2020) are important studies that show that vaccine efficacy is determined not only by the induction of tumour-specific T cells but also by the recruitment of myeloid effector cells by these vaccine-induced T cells, which are required to obtain full tumour cell growth control.
van Elsas, M. et al. Host genetics and tumor environment determine the functional impact of neutrophils in mouse tumor models. J Immunother Cancer 8, e000877 (2020).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Welters, M. J. P. et al. Intratumoral HPV16-specific t cells constitute a type I-oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin. Cancer Res. 24, 634–647 (2018).
Prat, A. et al. Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 77, 3540–3550 (2017).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Liu, Z. et al. Novel effector phenotype of Tim-3+ regulatory t cells leads to enhanced suppressive function in head and neck cancer patients. Clin. Cancer Res. 24, 4529–4538 (2018).
Santegoets, S. J. et al. Tbet-positive regulatory T cells accumulate in oropharyngeal cancers with ongoing tumor-specific type 1 T cell responses. J. Immunother. Cancer 7, 14 (2019).
Matoba, T. et al. Regulatory T cells expressing abundant CTLA-4 on the cell surface with a proliferative gene profile are key features of human head and neck cancer. Int. J. Cancer 144, 2811–2822 (2019).
Si, Y. et al. Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue. Sci Immunol 4, eaaw9159 (2019).
Oh, S. A. et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 1, 681–691 (2020).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 20, 1231–1243 (2019). The authors make the argument that for the best outcome ICI therapy should be administered after vaccination.
Germano, G. et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 552, 116–120 (2017).
Sharabi, A. B., Tran, P. T., Lim, M., Drake, C. G. & Deweese, T. L. Stereotactic radiation therapy combined with immunotherapy: augmenting the role of radiation in local and systemic treatment. Oncology 29, 331–340 (2015).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Ma, L. et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019).
Akahori, Y. et al. Antitumor activity of CAR-T cells targeting the intracellular oncoprotein WT1 can be enhanced by vaccination. Blood 132, 1134–1145 (2018).
Forde, P. M. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378, 1976–1986 (2018).
Provencio-Pulla, M. et al. Neoadjuvant chemo/immunotherapy for the treatment of stages IIIA resectable non-small cell lung cancer (NSCLC): a phase II multicenter exploratory study — NADIM study-SLCG. J. Clin. Oncol. 36, 8521–8521 (2018).
Topalian, S. L. et al. Neoadjuvant nivolumab for patients with resectable merkel cell carcinoma in the CheckMate 358 trial. Journal of Clinical Oncology 38, 2476–2487 (2020).
Nalley, C. I-SPY 2 trial explores immune checkpoint plus PARP inhibitors in breast cancer. Oncol. Times 42, 37 (2020).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Abdul Sater, H. et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J Immunother Cancer 8, e000655 (2020).
Subudhi, S. K. et al. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci Transl Med 12, eaaz3577 (2020).
Kranich, J. & Krautler, N. J. How follicular dendritic cells shape the B-cell antigenome. Front. Immunol. 7, 225 (2016).
Sautes-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Ahrends, T. et al. CD4+ T cell help confers a cytotoxic t cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 47, 848–861 e845 (2017).
Quezada, S. A. et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207, 637–650 (2010).
Bogen, B., Fauskanger, M., Haabeth, O. A. & Tveita, A. CD4+ T cells indirectly kill tumor cells via induction of cytotoxic macrophages in mouse models. Cancer Immunol. Immunother. 68, 1865–1873 (2019).
Sledzinska, A. et al. Regulatory T cells restrain interleukin-2- and Blimp-1-dependent acquisition of cytotoxic function by CD4+ T cells. Immunity 52, 151–166 e156 (2020).
Huffman, A. P., Lin, J. H., Kim, S. I., Byrne, K. T. & Vonderheide, R. H. CCL5 mediates CD40-driven CD4+ T cell tumor infiltration and immunity. JCI Insight 5, e137263 (2020).
Greyer, M. et al. T cell help amplifies innate signals in CD8+ DCs for optimal CD8+ T cell priming. Cell Rep. 14, 586–597 (2016).
Jiao, S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190 e1113 (2019).
Bos, R. & Sherman, L. A. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 70, 8368–8377 (2010).
Kishton, R. J., Sukumar, M. & Restifo, N. P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 26, 94–109 (2017).
Restifo, N. P. & Gattinoni, L. Lineage relationship of effector and memory T cells. Curr. Opin. Immunol. 25, 556–563 (2013).
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013 e1020 (2018).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37, 457–495 (2019).
Crespo, J., Sun, H., Welling, T. H., Tian, Z. & Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25, 214–221 (2013).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Vodnala, S. K. et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363, eaau0135 (2019).
Sukumar, M., Kishton, R. J. & Restifo, N. P. Metabolic reprograming of anti-tumor immunity. Curr. Opin. Immunol. 46, 14–22 (2017).
Yin, Z. et al. Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. J. Exp. Clin. Cancer Res. 38, 403 (2019).
Scharping, N. E. et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45, 701–703 (2016).
Teijeira, A. et al. Metabolic consequences of T-cell costimulation in anticancer immunity. Cancer Immunol. Res. 7, 1564–1569 (2019).
Acknowledgements
N.B. receives grants/research support from the NIH (R01 CA249175, R01 CA201189 and R01 AI081848), the Melanoma Research Alliance, the Cancer Research Institute, the Kimberly and Eric J. Waldman Department of Dermatology at the Icahn School of Medicine, the Ludwig Institute for Cancer Research, the Leukemia and Lymphoma Society, the Pershing Square Foundation, Regeneron Pharmaceuticals Inc., Dragonfly Therapeutics Inc. and Harbour Biomed Sciences.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.J.M.M. is Chief Scientific Officer of ISA Pharmaceuticals in Leiden, Netherlands, a biotechology company aiming at commercial development of synthetic peptide-based therapeutic vaccines against cancers caused by high-risk human papillomaviruses and against non-viral cancers. He receives a salary as a full-time employee at ISA Pharmaceuticals and is a beneficiary of a management participation plan that goes into effect if the company reaches a predefined value inflection point in the future. C.J.M.M. is an inventor on several patents regarding the use of synthetic long peptides as therapeutic vaccines for treatment of premalignant and malignant lesions. S.H.v.d.B.is named as an inventor on a patent for the use of synthetic long peptides as a vaccine, serves as a paid member of the strategy board of ISA Pharmaceuticals and received honoraria as a consultant for PCI Biotech, IO Biotech and DC prime, which develop cancer vaccines. N.B. serves as an advisor/board member for Neon Therapeutics, Novartis, Avidea, Boehringer Ingelheim, Rome Therapeutics, Roswell Park Comprehensive Cancer Center, MD Anderson Cancer Center, BreakBio, Carisma Therapeutics, CureVac, Genotwin, BioNTech, Gilead and Tempest Therapeutics. N.B. is an extramural member of the Parker Institute of Cancer Immunotherapy. M.S. declares no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks D. Speiser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Tumor Neoantigen Selection Alliance: https://www.parkerici.org/research-project/tumor-neoantigen-selection-alliance-tesla/
Glossary
- Immunogenic cell death
-
(ICD). A type of cell death that entails the release of danger-associated molecular patterns to attract and activate immune cells and the release of antigens to be acquired by activated antigen-presenting cells and presented to T cells.
- Danger-associated molecular patterns
-
Non-microbial endogenous factors released from dead or distressed cells that serve as activating ligands for innate immune receptors on antigen-presenting cells, prompting activation of antigen-presenting cells and secretion of cytokines and chemokines to recruit other immune cells.
- Antigen cross-presentation
-
A specialized mechanism that allows select cells such as type 1 dendritic cells to process and present internalized exogenous antigens on MHC class I molecules to activate CD8+ T cells.
- Sipuleucil-T
-
Dendritic cell-focused cell-based vaccine for hormone-refractory prostate cancer consisting of a prostate acid phosphatase, fused with GM-CSF, loaded ex vivo on blood cells partially enriched for dendritic cells.
- ‘Cold’ tumours
-
Non-inflamed tumours lacking the presence of immune cells, particularly cytotoxic T cells, in the tumour bed and the invasive margin.
- Antigen spreading
-
A phenomenon where endogenous cellular immune responses develop against epitopes not targeted by the vaccine or immunotherapy.
- Stimulator of interferon genes protein
-
(STING). An intracellular pattern recognition receptor activated by double-stranded DNA, cyclic GMP–AMP and microbial cyclic dinucleotides to induce a strong interferon response.
- Talimogene laherparepvec
-
(TVec). An oncolytic virus therapy composed of herpes simplex virus type 1 modified to infect tumour cells and express GM-CSF, a cytokine known for promoting differentiation of dendritic cells.
Rights and permissions
About this article
Cite this article
Saxena, M., van der Burg, S.H., Melief, C.J.M. et al. Therapeutic cancer vaccines. Nat Rev Cancer 21, 360–378 (2021). https://doi.org/10.1038/s41568-021-00346-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00346-0
This article is cited by
-
Revealing the role of SPP1+ macrophages in glioma prognosis and therapeutic targeting by investigating tumor-associated macrophage landscape in grade 2 and 3 gliomas
Cell & Bioscience (2024)
-
The application of nanoparticles-based ferroptosis, pyroptosis and autophagy in cancer immunotherapy
Journal of Nanobiotechnology (2024)
-
Vaccines: a promising therapy for myelodysplastic syndrome
Journal of Hematology & Oncology (2024)
-
Roles of exosomes in immunotherapy for solid cancers
Cell Death & Disease (2024)
-
How does a cancer vaccine work?
Nature (2024)