Abstract
Diffuse large B cell lymphoma (DLBCL) is a highly heterogeneous lymphoid neoplasm with variations in gene expression profiles and genetic alterations, which lead to substantial variations in clinical course and response to therapy. The advent of high-throughput genome sequencing platforms, and especially whole-exome sequencing, has helped to define the genetic landscape of DLBCL. In the past 10 years, these studies have identified many genetic alterations in DLBCL, some of which are specific to B cell lymphomas, whereas others can also be observed in other types of cancer. These aberrations result in altered activation of a wide range of signalling pathways and other cellular processes, including those involved in B cell differentiation, B cell receptor signalling, activation of the NF-κB pathway, apoptosis and epigenetic regulation. Further elaboration of the genetics of DLBCL will not only improve our understanding of disease pathogenesis but also provide further insight into disease classification, prognostication and therapeutic targets. In this Review, we describe the current understanding of the prevalence and causes of specific genetic alterations in DLBCL and their role in disease development and progression. We also summarize the available clinical data on therapies designed to target the aberrant pathways driven by these alterations.
Key points
-
Application of next-generation sequencing technologies, especially whole-exome sequencing, has helped to define the genomic landscape of diffuse large B cell lymphoma (DLBCL).
-
Characterization of genetic events provides important insights into the pathogenesis of DLBCL.
-
The genetic events identified in DLBCL have prognostic implications and can also enable the molecular classification of DLBCL into specific subtypes.
-
Novel agents have been developed to target the dysregulated signalling pathways caused by genetic events in DLBCL, and some of these agents have achieved promising efficacies.
-
Several genetic biomarkers are predictive of a response to novel targeted agents in patients with DLBCL and could be used in the future to guide patient selection for clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Swerdlow, S. H. et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (IARC Press, 2017).
Feugier, P. et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 23, 4117–4126 (2005).
Pfreundschuh, M. et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 9, 105–116 (2008).
Pfreundschuh, M. et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 7, 379–391 (2006).
Coiffier, B. & Sarkozy, C. Diffuse large B cell lymphoma: R-CHOP failure-what to do? Hematol. Am. Soc. Hematol. Educ. Program 2016, 366–378 (2016).
Gisselbrecht, C. et al. Salvage regimens with autologous transplantation for relapsed large B cell lymphoma in the rituximab era. J. Clin. Oncol. 28, 4184–4190 (2010).
Friedberg, J. W. Relapsed/refractory diffuse large B cell lymphoma. Hematol. Am. Soc. Hematol. Educ. Program 2011, 498–505 (2011).
Alizadeh, A. A. et al. Distinct types of diffuse large B cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000).
Xu-Monette, Z. Y. et al. Assessment of CD37 B cell antigen and cell of origin significantly improves risk prediction in diffuse large B cell lymphoma. Blood 128, 3083–3100 (2016).
Gutierrez-Garcia, G. et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B cell lymphoma treated with immunochemotherapy. Blood 117, 4836–4843 (2011).
Reddy, A. et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell 171, 481–494 (2017).
Schmitz, R. et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 378, 1396–1407 (2018).
Chapuy, B. et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 24, 679–690 (2018).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Jager, U. et al. Follicular lymphomas’ BCL-2/IgH junctions contain templated nucleotide insertions: novel insights into the mechanism of t(14;18) translocation. Blood 95, 3520–3529 (2000).
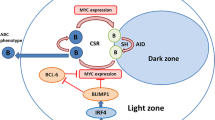
Shen, H. M., Peters, A., Baron, B., Zhu, X. & Storb, U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science 280, 1750–1752 (1998).
Lieber, M. R. Mechanisms of human lymphoid chromosomal translocations. Nat. Rev. Cancer 16, 387–398 (2016).
Lenz, G. et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J. Exp. Med. 204, 633–643 (2007).
Deutsch, A. J. et al. MALT lymphoma and extranodal diffuse large B cell lymphoma are targeted by aberrant somatic hypermutation. Blood 109, 3500–3504 (2007).
Gordon, M. S., Kanegai, C. M., Doerr, J. R. & Wall, R. Somatic hypermutation of the B cell receptor genes B29 (Igbeta, CD79b) and mb1 (Igalpha, CD79a). Proc. Natl Acad. Sci. USA 100, 4126–4131 (2003).
Pasqualucci, L. et al. Hypermutation of multiple proto-oncogenes in B cell diffuse large-cell lymphomas. Nature 412, 341–346 (2001).
De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015).
Basso, K. & Dalla-Favera, R. Germinal centres and B cell lymphomagenesis. Nat. Rev. Immunol. 15, 172–184 (2015).
Hatzi, K. et al. A hybrid mechanism of action for BCL6 in B cells defined by formation of functionally distinct complexes at enhancers and promoters. Cell Rep. 4, 578–588 (2013).
Ye, B. H. et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 262, 747–750 (1993).
Ye, B. H. et al. Chromosomal translocations cause deregulated BCL6 expression by promoter substitution in B cell lymphoma. EMBO J. 14, 6209–6217 (1995).
Ye, Q. et al. Prognostic impact of concurrent MYC and BCL6 rearrangements and expression in de novo diffuse large B cell lymphoma. Oncotarget 7, 2401–2416 (2016).
Tibiletti, M. G. et al. BCL2, BCL6, MYC, MALT 1, and BCL10 rearrangements in nodal diffuse large B cell lymphomas: a multicenter evaluation of a new set of fluorescent in situ hybridization probes and correlation with clinical outcome. Hum. Pathol. 40, 645–652 (2009).
Iqbal, J. et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B cell lymphoma. Leukemia 21, 2332–2343 (2007).
Pasqualucci, L. et al. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B cell lymphoma. Blood 101, 2914–2923 (2003).
Ying, C. Y. et al. MEF2B mutations lead to deregulated expression of the oncogene BCL6 in diffuse large B cell lymphoma. Nat. Immunol. 14, 1084–1092 (2013).
Duan, S. et al. FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B cell lymphomas. Nature 481, 90–93 (2012).
Pasqualucci, L. et al. Inactivating mutations of acetyltransferase genes in B cell lymphoma. Nature 471, 189–195 (2011).
Cattoretti, G. et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell 7, 445–455 (2005).
Shaffer, A. L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Pasqualucci, L. et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J. Exp. Med. 203, 311–317 (2006).
Mandelbaum, J. et al. BLIMP1 is a tumor suppressor gene frequently disrupted in activated B cell-like diffuse large B cell lymphoma. Cancer Cell 18, 568–579 (2010).
Calado, D. P. et al. Constitutive canonical NF-kappaB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer Cell 18, 580–589 (2010).
Rawlings, D. J., Metzler, G., Wray-Dutra, M. & Jackson, S. W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 17, 421–436 (2017).
Davis, R. E. et al. Chronic active B cell-receptor signalling in diffuse large B cell lymphoma. Nature 463, 88–92 (2010).
Young, R. M. & Staudt, L. M. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat. Rev. Drug Discov. 12, 229–243 (2013).
Young, R. M., Shaffer, A. L. 3rd, Phelan, J. D. & Staudt, L. M. B cell receptor signaling in diffuse large B cell lymphoma. Semin. Hematol. 52, 77–85 (2015).
Havranek, O. et al. Tonic B cell receptor signaling in diffuse large B cell lymphoma. Blood 130, 995–1006 (2017).
Lenz, G. et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319, 1676–1679 (2008).
Bohers, E. et al. Targetable activating mutations are very frequent in GCB and ABC diffuse large B cell lymphoma. Genes Chromosomes Cancer 53, 144–153 (2014).
Lamason, R. L., McCully, R. R., Lew, S. M. & Pomerantz, J. L. Oncogenic CARD11 mutations induce hyperactive signaling by disrupting autoinhibition by the PKC-responsive inhibitory domain. Biochemistry 49, 8240–8250 (2010).
Liu, H. et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 98, 1182–1187 (2001).
Willis, T. G. et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 96, 35–45 (1999).
Nagel, D. et al. Pharmacologic inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell 22, 825–837 (2012).
Ngo, V. N. et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 (2011).
Dubois, S. et al. Biological and clinical relevance of associated genomic alterations in MYD88 L265P and non-L265P-mutated diffuse large B-cell lymphoma: analysis of 361 cases. Clin. Cancer Res. 23, 2232–2244 (2017).
Rovira, J. et al. MYD88 L265P mutations, but no other variants, identify a subpopulation of DLBCL patients of activated B cell origin, extranodal involvement, and poor outcome. Clin. Cancer Res. 22, 2755–2764 (2016).
Montesinos-Rongen, M. et al. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 122, 791–792 (2011).
Zheng, M. et al. Frequency of MYD88 and CD79B mutations, and MGMT methylation in primary central nervous system diffuse large B cell lymphoma. Neuropathology 37, 509–516 (2017).
Fukumura, K. et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol. 131, 865–875 (2016).
Chapuy, B. et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 127, 869–881 (2016).
Taniguchi, K. et al. Frequent MYD88 L265P and CD79B mutations in primary breast diffuse large B-cell lymphoma. Am. J. Surg. Pathol. 40, 324–334 (2016).
Cao, X. X. et al. Patients with primary breast and primary female genital tract diffuse large B cell lymphoma have a high frequency of MYD88 and CD79B mutations. Ann. Hematol. 96, 1867–1871 (2017).
Franco, F. et al. Mutational profile of primary breast diffuse large B cell lymphoma. Oncotarget 8, 102888–102897 (2017).
Pham-Ledard, A. et al. MYD88 somatic mutation is a genetic feature of primary cutaneous diffuse large B cell lymphoma, leg type. J. Invest. Dermatol. 132, 2118–2120 (2012).
Pham-Ledard, A. et al. Multiple genetic alterations in primary cutaneous large B cell lymphoma, leg type support a common lymphomagenesis with activated B cell-like diffuse large B cell lymphoma. Mod. Pathol. 27, 402–411 (2014).
Pham-Ledard, A. et al. High frequency and clinical prognostic value of MYD88 L265P mutation in primary cutaneous diffuse large B cell lymphoma, leg-type. JAMA Dermatol. 150, 1173–1179 (2014).
Zhou, X. A. et al. Genomic analyses identify recurrent alterations in immune evasion genes in diffuse large B-cell lymphoma, leg type. J. Invest. Dermatol. 138, 2365–2376 (2018).
Kraan, W. et al. High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B cell lymphoma. Leukemia 28, 719–720 (2014).
Oishi, N. et al. High prevalence of the MYD88 mutation in testicular lymphoma: Immunohistochemical and genetic analyses. Pathol. Int. 65, 528–535 (2015).
Phelan, J. D. et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 560, 387–391 (2018).
Gilmore, T. D., Kalaitzidis, D., Liang, M. C. & Starczynowski, D. T. The c-Rel transcription factor and B cell proliferation: a deal with the devil. Oncogene 23, 2275–2286 (2004).
Kaltschmidt, B., Greiner, J. F. W., Kadhim, H. M. & Kaltschmidt, C. Subunit-specific role of NF-kappaB in cancer. Biomedicines 6, E44 (2018).
Li, L. et al. Prognostic impact of c-Rel nuclear expression and REL amplification and crosstalk between c-Rel and the p53 pathway in diffuse large B cell lymphoma. Oncotarget 6, 23157–23180 (2015).
Ma, A. & Malynn, B. A. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 12, 774–785 (2012).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430, 694–699 (2004).
Compagno, M. et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B cell lymphoma. Nature 459, 717–721 (2009).
Wang, J. Q., Jeelall, Y. S., Beutler, B., Horikawa, K. & Goodnow, C. C. Consequences of the recurrent MYD88(L265P) somatic mutation for B cell tolerance. J. Exp. Med. 211, 413–426 (2014).
Wenzl, K. et al. Loss of TNFAIP3 enhances MYD88L265P-driven signaling in non-Hodgkin lymphoma. Blood Cancer J. 8, 97 (2018).
Uddin, S. et al. Role of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large B cell lymphoma survival. Blood 108, 4178–4186 (2006).
Zhang, J. et al. Genetic heterogeneity of diffuse large B cell lymphoma. Proc. Natl Acad. Sci. USA 110, 1398–1403 (2013).
Wang, X. et al. Clinical significance of PTEN deletion, mutation, and loss of PTEN expression in de novo diffuse large B-cell lymphoma. Neoplasia 20, 574–593 (2018).
Lenz, G. et al. Molecular subtypes of diffuse large B cell lymphoma arise by distinct genetic pathways. Proc. Natl Acad. Sci. USA 105, 13520–13525 (2008).
Pfeifer, M. et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B cell lymphoma. Proc. Natl Acad. Sci. USA 110, 12420–12425 (2013).
Jardin, F. et al. Diffuse large B cell lymphomas with CDKN2A deletion have a distinct gene expression signature and a poor prognosis under R-CHOP treatment: a GELA study. Blood 116, 1092–1104 (2010).
Xu-Monette, Z. Y. et al. Dysfunction of the TP53 tumor suppressor gene in lymphoid malignancies. Blood 119, 3668–3683 (2012).
Xu-Monette, Z. Y. et al. Mutational profile and prognostic significance of TP53 in diffuse large B cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood 120, 3986–3996 (2012).
Li, Y. et al. Single nucleotide variation in the TP53 3’ untranslated region in diffuse large B cell lymphoma treated with rituximab-CHOP: a report from the International DLBCL Rituximab-CHOP Consortium Program. Blood 121, 4529–4540 (2013).
Monti, S. et al. Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell 22, 359–372 (2012).
Xu-Monette, Z. Y. et al. MDM2 phenotypic and genotypic profiling, respective to TP53 genetic status, in diffuse large B cell lymphoma patients treated with rituximab-CHOP immunochemotherapy: a report from the International DLBCL Rituximab-CHOP Consortium Program. Blood 122, 2630–2640 (2013).
Karube, K. & Campo, E. MYC alterations in diffuse large B cell lymphomas. Semin. Hematol. 52, 97–106 (2015).
Copie-Bergman, C. et al. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood 126, 2466–2474 (2015).
Stasik, C. J. et al. Increased MYC gene copy number correlates with increased mRNA levels in diffuse large B cell lymphoma. Haematologica 95, 597–603 (2010).
Quesada, A. E. et al. Increased MYC copy number is an independent prognostic factor in patients with diffuse large B cell lymphoma. Mod. Pathol. 30, 1688–1697 (2017).
Ennishi, D. et al. Genetic profiling of MYC and BCL2 in diffuse large B cell lymphoma determines cell-of-origin-specific clinical impact. Blood 129, 2760–2770 (2017).
Xu-Monette, Z. Y. et al. Clinical and Biologic Significance of MYC Genetic Mutations in De Novo Diffuse Large B cell Lymphoma. Clin. Cancer Res. 22, 3593–3605 (2016).
Visco, C. et al. Patients with diffuse large B cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica 98, 255–263 (2013).
Iqbal, J. et al. BCL2 translocation defines a unique tumor subset within the germinal center B cell-like diffuse large B cell lymphoma. Am. J. Pathol. 165, 159–166 (2004).
Kridel, R., Sehn, L. H. & Gascoyne, R. D. Pathogenesis of follicular lymphoma. J. Clin. Invest. 122, 3424–3431 (2012).
Monni, O. et al. BCL2 overexpression associated with chromosomal amplification in diffuse large B cell lymphoma. Blood 90, 1168–1174 (1997).
Rantanen, S., Monni, O., Joensuu, H., Franssila, K. & Knuutila, S. Causes and consequences of BCL2 overexpression in diffuse large B cell lymphoma. Leuk. Lymphoma 42, 1089–1098 (2001).
Kusumoto, S. et al. Diffuse large B cell lymphoma with extra Bcl-2 gene signals detected by FISH analysis is associated with a “non-germinal center phenotype”. Am. J. Surg. Pathol. 29, 1067–1073 (2005).
Schuetz, J. M. et al. BCL2 mutations in diffuse large B cell lymphoma. Leukemia 26, 1383–1390 (2012).
Saito, M. et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc. Natl Acad. Sci. USA 106, 11294–11299 (2009).
Monaco, G. et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 19, 295–309 (2012).
Deng, X., Gao, F., Flagg, T., Anderson, J. & May, W. S. Bcl2’s flexible loop domain regulates p53 binding and survival. Mol. Cell. Biol. 26, 4421–4434 (2006).
Grandgirard, D. et al. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 17, 1268–1278 (1998).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Lee, S. Y. et al. Gain-of-function mutations and copy number increases of Notch2 in diffuse large B cell lymphoma. Cancer Sci. 100, 920–926 (2009).
Arcaini, L. et al. The NOTCH pathway is recurrently mutated in diffuse large B cell lymphoma associated with hepatitis C virus infection. Haematologica 100, 246–252 (2015).
Green, J. A. et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat. Immunol. 12, 672–680 (2011).
Muppidi, J. R., Lu, E. & Cyster, J. G. The G protein-coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination. J. Exp. Med. 212, 2213–2222 (2015).
Muppidi, J. R. et al. Loss of signalling via Galpha13 in germinal centre B cell-derived lymphoma. Nature 516, 254–258 (2014).
Morin, R. D. et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476, 298–303 (2011).
Kirmizis, A. et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 18, 1592–1605 (2004).
Caganova, M. et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J. Clin. Invest. 123, 5009–5022 (2013).
Velichutina, I. et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood 116, 5247–5255 (2010).
Beguelin, W. et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23, 677–692 (2013).
Morin, R. D. et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B cell lymphomas of germinal-center origin. Nat. Genet. 42, 181–185 (2010).
Sneeringer, C. J. et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B cell lymphomas. Proc. Natl Acad. Sci. USA 107, 20980–20985 (2010).
Yap, D. B. et al. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood 117, 2451–2459 (2011).
Sahasrabuddhe, A. A. et al. Oncogenic Y641 mutations in EZH2 prevent Jak2/beta-TrCP-mediated degradation. Oncogene 34, 445–454 (2015).
Ortega-Molina, A. et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat. Med. 21, 1199–1208 (2015).
Zhang, J. et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat. Med. 21, 1190–1198 (2015).
Pasqualucci, L. et al. Analysis of the coding genome of diffuse large B cell lymphoma. Nat. Genet. 43, 830–837 (2011).
Lohr, J. G. et al. Discovery and prioritization of somatic mutations in diffuse large B cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl Acad. Sci. USA 109, 3879–3884 (2012).
Horton, S. J. et al. Early loss of Crebbp confers malignant stem cell properties on lymphoid progenitors. Nat. Cell Biol. 19, 1093–1104 (2017).
Zhang, J. et al. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in B cell lymphoma. Cancer Discov. 7, 322–337 (2017).
Hashwah, H. et al. Inactivation of CREBBP expands the germinal center B cell compartment, down-regulates MHCII expression and promotes DLBCL growth. Proc. Natl Acad. Sci. USA 114, 9701–9706 (2017).
Jiang, Y. et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov. 7, 38–53 (2017).
Grossman, S. R. p300/CBP/p53 interaction and regulation of the p53 response. Eur. J. Biochem. 268, 2773–2778 (2001).
Challa-Malladi, M. et al. Combined genetic inactivation of beta2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell 20, 728–740 (2011).
Steimle, V., Siegrist, C. A., Mottet, A., Lisowska-Grospierre, B. & Mach, B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 265, 106–109 (1994).
Georgiou, K. et al. Genetic basis of PD-L1 overexpression in diffuse large B cell lymphomas. Blood 127, 3026–3034 (2016).
Kataoka, K. et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 534, 402–406 (2016).
Wang, X. et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal 27, 443–452 (2015).
Cortez, M. A. et al. PDL1 regulation by p53 via miR-34. J. Natl Cancer Inst. 108, djv303 (2016).
Boice, M. et al. Loss of the HVEM tumor suppressor in lymphoma and restoration by modified CAR-T cells. Cell 167, 405–418 (2016).
Young, K. H. et al. Structural profiles of TP53 gene mutations predict clinical outcome in diffuse large B cell lymphoma: an international collaborative study. Blood 112, 3088–3098 (2008).
Young, K. H. et al. Mutations in the DNA-binding codons of TP53, which are associated with decreased expression of TRAILreceptor-2, predict for poor survival in diffuse large B cell lymphoma. Blood 110, 4396–4405 (2007).
Zenz, T. et al. TP53 mutation and survival in aggressive B cell lymphoma. Int. J. Cancer 141, 1381–1388 (2017).
Wang, X. J. et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B cell lymphoma. Mod. Pathol. 30, 194–203 (2017).
Jia, Z. et al. P53 deletion is independently associated with increased age and decreased survival in a cohort of Chinese patients with diffuse large B cell lymphoma. Leuk. Lymphoma 53, 2182–2185 (2012).
Cao, Y. et al. Mutations or copy number losses of CD58 and TP53 genes in diffuse large B cell lymphoma are independent unfavorable prognostic factors. Oncotarget 7, 83294–83307 (2016).
Le Gouill, S. et al. The clinical presentation and prognosis of diffuse large B cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 92, 1335–1342 (2007).
Klapper, W. et al. Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Leukemia 22, 2226–2229 (2008).
Niitsu, N., Okamoto, M., Miura, I. & Hirano, M. Clinical features and prognosis of de novo diffuse large B cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 23, 777–783 (2009).
Barrans, S. et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B cell lymphoma treated in the era of rituximab. J. Clin. Oncol. 28, 3360–3365 (2010).
Kuhnl, A. et al. Outcome of elderly patients with diffuse large B cell lymphoma treated with R-CHOP: results from the UK NCRI R-CHOP14v21 trial with combined analysis of molecular characteristics with the DSHNHL RICOVER-60 trial. Ann. Oncol. 28, 1540–1546 (2017).
Cuccuini, W. et al. MYC+ diffuse large B cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood 119, 4619–4624 (2012).
Lai, C. et al. MYC gene rearrangement in diffuse large B cell lymphoma does not confer a worse prognosis following dose-adjusted EPOCH-R. Leuk. Lymphoma 59, 505–508 (2018).
Landsburg, D. J. et al. Sole rearrangement but not amplification of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma and B cell lymphoma unclassifiable. Br. J. Haematol. 175, 631–640 (2016).
Testoni, M. et al. Gains of MYC locus and outcome in patients with diffuse large B cell lymphoma treated with R-CHOP. Br. J. Haematol. 155, 274–277 (2011).
Lu, T. X. et al. MYC or BCL2 copy number aberration is a strong predictor of outcome in patients with diffuse large B cell lymphoma. Oncotarget 6, 18374–18388 (2015).
Juskevicius, D. et al. Mutations of CREBBP and SOCS1 are independent prognostic factors in diffuse large B cell lymphoma: mutational analysis of the SAKK 38/07 prospective clinical trial cohort. J. Hematol. Oncol. 10, 70 (2017).
Dubois, S. et al. Next-generation sequencing in diffuse large B-cell lymphoma highlights molecular divergence and therapeutic opportunities: a LYSA study. Clin. Cancer Res. 22, 2919–2928 (2016).
Scott, D. W. et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J. Clin. Oncol. 33, 2848–2856 (2015).
Hu, S. et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B cell subtype of diffuse large B cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 121, 4021–4031 (2013).
Zhang, L. H. et al. Lenalidomide efficacy in activated B cell-like subtype diffuse large B cell lymphoma is dependent upon IRF4 and cereblon expression. Br. J. Haematol. 160, 487–502 (2013).
Hernandez-Ilizaliturri, F. J. et al. Higher response to lenalidomide in relapsed/refractory diffuse large B cell lymphoma in nongerminal center B cell-like than in germinal center B cell-like phenotype. Cancer 117, 5058–5066 (2011).
Wiernik, P. H. et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 26, 4952–4957 (2008).
Witzig, T. E. et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B cell non-Hodgkin’s lymphoma. Ann. Oncol. 22, 1622–1627 (2011).
Zinzani, P. L. et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B cell lymphoma: a phase 2 trial. Clin. Lymphoma Myeloma Leuk. 11, 462–466 (2011).
Wang, M. et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia 27, 1902–1909 (2013).
Feldman, T. et al. Addition of lenalidomide to rituximab, ifosfamide, carboplatin, etoposide (RICER) in first-relapse/primary refractory diffuse large B cell lymphoma. Br. J. Haematol. 166, 77–83 (2014).
Martin, A. et al. Lenalidomide in combination with R-ESHAP in patients with relapsed or refractory diffuse large B cell lymphoma: a phase 1b study from GELTAMO group. Br. J. Haematol. 173, 245–252 (2016).
Hitz, F. et al. Rituximab, bendamustine, and lenalidomide in patients with aggressive B cell lymphoma not eligible for high-dose chemotherapy or anthracycline-based therapy: phase I results of the SAKK 38/08 trial. Ann. Hematol. 92, 1033–1040 (2013).
Hitz, F. et al. Rituximab, bendamustine and lenalidomide in patients with aggressive B cell lymphoma not eligible for anthracycline-based therapy or intensive salvage chemotherapy - SAKK 38/08. Br. J. Haematol. 174, 255–263 (2016).
Ferreri, A. J. et al. Lenalidomide maintenance in patients with relapsed diffuse large B cell lymphoma who are not eligible for autologous stem cell transplantation: an open label, single-arm, multicentre phase 2 trial. Lancet Haematol. 4, e137–e146 (2017).
Czuczman, M. S. et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin. Cancer Res. 23, 4127–4137 (2017).
Nowakowski, G. S. et al. Lenalidomide can be safely combined with R-CHOP (R2CHOP) in the initial chemotherapy for aggressive B cell lymphomas: phase I study. Leukemia 25, 1877–1881 (2011).
Chiappella, A. et al. Lenalidomide plus cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab is safe and effective in untreated, elderly patients with diffuse large B cell lymphoma: a phase I study by the Fondazione Italiana Linfomi. Haematologica 98, 1732–1738 (2013).
Vitolo, U. et al. Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol. 15, 730–737 (2014).
Nowakowski, G. S. et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J. Clin. Oncol. 33, 251–257 (2015).
Nowakowski, G. S. et al. ROBUST: Lenalidomide-R-CHOP versus placebo-R-CHOP in previously untreated ABC-type diffuse large B cell lymphoma. Future Oncol. 12, 1553–1563 (2016).
Thieblemont, C. et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 35, 2473–2481 (2017).
Reddy, N. M. et al. A phase II randomized study of lenalidomide or lenalidomide and rituximab as maintenance therapy following standard chemotherapy for patients with high/high-intermediate risk diffuse large B cell lymphoma. Leukemia 31, 241–244 (2017).
Chen, D., Frezza, M., Schmitt, S., Kanwar, J. & Dou, Q. P. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr. Cancer Drug Targets 11, 239–253 (2011).
Goy, A. et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 23, 667–675 (2005).
Furman, R. R. et al. Phase 1 trial of bortezomib plus R-CHOP in previously untreated patients with aggressive non-Hodgkin lymphoma. Cancer 116, 5432–5439 (2010).
Dunleavy, K. et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B cell lymphoma. Blood 113, 6069–6076 (2009).
Evens, A. M. et al. A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T cell lymphomas. Br. J. Haematol. 163, 55–61 (2013).
Ruan, J. et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B cell lymphoma and mantle cell lymphoma. J. Clin. Oncol. 29, 690–697 (2011).
Offner, F. et al. Frontline rituximab, cyclophosphamide, doxorubicin, and prednisone with bortezomib (VR-CAP) or vincristine (R-CHOP) for non-GCB DLBCL. Blood 126, 1893–1901 (2015).
Leonard, J. P. et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J. Clin. Oncol. 35, 3538–3546 (2017).
Wilson, W. H. et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 21, 922–926 (2015).
Younes, A. et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 15, 1019–1026 (2014).
Sauter, C. S. et al. A phase 1 study of ibrutinib in combination with R-ICE in patients with relapsed or primary refractory DLBCL. Blood 131, 1805–1808 (2018).
Ramchandren, R. et al. The iR2 regimen (ibrutinib, lenalidomide, and rituximab) is active with a manageable safety profile in patients with relapsed/refractory non-germinal center-like diffuse large B-cell lymphoma. Blood 132, S402 (2018).
Younes, A. et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J. Clin. Oncol. https://doi.org/10.1200/JCO.18.02403 (2019).
Grommes, C. et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 7, 1018–1029 (2017).
Lionakis, M. S. et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell 31, 833–843 (2017).
Grommes, C. et al. Phase Ib trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood https://doi.org/10.1182/blood-2018-09-875732 (2018).
Robertson, M. J. et al. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B cell lymphoma. J. Clin. Oncol. 25, 1741–1746 (2007).
Crump, M. et al. Randomized, double-blind, phase III trial of enzastaurin versus placebo in patients achieving remission after first-line therapy for high-risk diffuse large B-cell lymphoma. J. Clin. Oncol. 34, 2484–2492 (2016).
Hainsworth, J. D. et al. A randomized, phase 2 study of R-CHOP plus enzastaurin versus R-CHOP in patients with intermediate- or high-risk diffuse large B cell lymphoma. Leuk. Lymphoma 57, 216–218 (2016).
Friedberg, J. W. et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115, 2578–2585 (2010).
Barr, P. M. et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood 127, 2411–2415 (2016).
Burke, J. M. et al. An open-label, phase II trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in diffuse large B cell lymphoma. Clin. Lymphoma Myeloma Leuk. 18, e327–e331 (2018).
Flinn, I. W. et al. A phase II trial to evaluate the efficacy of fostamatinib in patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL). Eur. J. Cancer 54, 11–17 (2016).
Brown, J. R. et al. Phase I trial of the pan-PI3K inhibitor pilaralisib (SAR245408/XL147) in patients with chronic lymphocytic leukemia (CLL) or relapsed/refractory lymphoma. Clin. Cancer Res. 21, 3160–3169 (2015).
Younes, A. et al. Pan-phosphatidylinositol 3-kinase inhibition with buparlisib in patients with relapsed or refractory non-Hodgkin lymphoma. Haematologica 102, 2104–2112 (2017).
Dreyling, M. et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann. Oncol. 28, 2169–2178 (2017).
Oki, Y. et al. CUDC-907 in relapsed/refractory diffuse large B cell lymphoma, including patients with MYC-alterations: results from an expanded phase I trial. Haematologica 102, 1923–1930 (2017).
Wang, J. et al. AKT hyperactivation and the potential of AKT-targeted therapy in diffuse large B-cell lymphoma. Am. J. Pathol. 187, 1700–1716 (2017).
Oki, Y. et al. Phase II study of an AKT inhibitor MK2206 in patients with relapsed or refractory lymphoma. Br. J. Haematol. 171, 463–470 (2015).
Witzig, T. E. et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia 25, 341–347 (2011).
Smith, S. M. et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: the University of Chicago phase II consortium. J. Clin. Oncol. 28, 4740–4746 (2010).
Barnes, J. A. et al. Everolimus in combination with rituximab induces complete responses in heavily pretreated diffuse large B cell lymphoma. Haematologica 98, 615–619 (2013).
Fenske, T. S. et al. A phase 2 study of weekly temsirolimus and bortezomib for relapsed or refractory B cell non-Hodgkin lymphoma: a Wisconsin Oncology Network study. Cancer 121, 3465–3471 (2015).
Witzig, T. E. et al. Adjuvant everolimus in high-risk diffuse large B cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann. Oncol. 29, 707–714 (2018).
Johnston, P. B. et al. Everolimus combined with R-CHOP-21 for new, untreated, diffuse large B cell lymphoma (NCCTG 1085 [Alliance]): safety and efficacy results of a phase 1 and feasibility trial. Lancet Haematol. 3, e309–e316 (2016).
Witzig, T. E. et al. High rate of event-free survival at 24 months with everolimus/RCHOP for untreated diffuse large B cell lymphoma: updated results from NCCTG N1085 (Alliance). Blood Cancer J. 7, e576 (2017).
Italiano, A. et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 19, 649–659 (2018).
Ribrag, V. et al. Interim results from an ongoing phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). Blood 132, 4196 (2018).
Sarkozy, C. et al. Results from a phase Ib evaluation of tazemetostat (EPZ-6438) in combination with R-CHOP in poor prognosis newly diagnosed diffuse large B cell lymphoma (DLBCL): a Lysa study. Blood 132, 4191 (2018).
Straus, D. J. et al. Phase I/II trial of vorinostat with rituximab, cyclophosphamide, etoposide and prednisone as palliative treatment for elderly patients with relapsed or refractory diffuse large B cell lymphoma not eligible for autologous stem cell transplantation. Br. J. Haematol. 168, 663–670 (2015).
Batlevi, C. L. et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br. J. Haematol. 178, 434–441 (2017).
Kelly, W. K. et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J. Clin. Oncol. 23, 3923–3931 (2005).
Crump, M. et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B cell lymphoma. Ann. Oncol. 19, 964–969 (2008).
Budde, L. E. et al. A phase I study of pulse high-dose vorinostat (V) plus rituximab (R), ifosphamide, carboplatin, and etoposide (ICE) in patients with relapsed lymphoma. Br. J. Haematol. 161, 183–191 (2013).
Assouline, S. E. et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B cell lymphoma. Blood 128, 185–194 (2016).
Oki, Y. et al. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin. Cancer Res. 19, 6882–6890 (2013).
Ribrag, V. et al. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: results of a phase II study. Haematologica 102, 903–909 (2017).
Holkova, B. et al. Phase 1 trial of carfilzomib (PR-171) in combination with vorinostat (SAHA) in patients with relapsed or refractory B cell lymphomas. Leuk. Lymphoma 57, 635–643 (2016).
Nieto, Y. et al. Double epigenetic modulation of high-dose chemotherapy with azacitidine and vorinostat for patients with refractory or poor-risk relapsed lymphoma. Cancer 122, 2680–2688 (2016).
Hofmeister, C. C. et al. A phase 1 study of vorinostat maintenance after autologous transplant in high-risk lymphoma. Leuk. Lymphoma 56, 1043–1049 (2015).
Nieto, Y. et al. Vorinostat combined with high-dose gemcitabine, busulfan, and melphalan with autologous stem cell transplantation in patients with refractory lymphomas. Biol. Blood Marrow Transplant 21, 1914–1920 (2015).
Davids, M. S. et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol. 35, 826–833 (2017).
de Vos, S. et al. Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase 1b dose-finding study. Ann. Oncol. 29, 1932–1938 (2018).
Morschhauser, F. et al. Venetoclax plus rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) improves outcomes in BCL2-positive first-line diffuse large B-cell lymphoma (DLBCL): first safety, efficacy and biomarker analyses from the phase II CAVALLI study. Blood 132, 782 (2018).
Liu, Y. et al. NOXA genetic amplification or pharmacologic induction primes lymphoma cells to BCL2 inhibitor-induced cell death. Proc. Natl Acad. Sci. USA 115, 12034–12039 (2018).
Amorim, S. et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 3, e196–e204 (2016).
Lesokhin, A. M. et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J. Clin. Oncol. 34, 2698–2704 (2016).
Ansell, S. M. et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J. Clin. Oncol. 37, 481–489 (2019).
Kline, J. et al. PD-L1 gene alterations identify a unique subset of diffuse large B cell lymphoma that harbors a T cell inflamed phenotype. Blood 132, 673 (2018).
Nayak, L. et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 129, 3071–3073 (2017).
Greenawalt, D. M. et al. Comparative analysis of primary versus relapse/refractory DLBCL identifies shifts in mutation spectrum. Oncotarget 8, 99237–99244 (2017).
Melchardt, T. et al. Clonal evolution in relapsed and refractory diffuse large B cell lymphoma is characterized by high dynamics of subclones. Oncotarget 7, 51494–51502 (2016).
Morin, R. D. et al. Genetic landscapes of relapsed and refractory diffuse large B-cell lymphomas. Clin. Cancer Res. 22, 2290–2300 (2016).
Mareschal, S. et al. Whole exome sequencing of relapsed/refractory patients expands the repertoire of somatic mutations in diffuse large B cell lymphoma. Genes Chromosomes Cancer 55, 251–267 (2016).
Park, H. Y. et al. Whole-exome and transcriptome sequencing of refractory diffuse large B cell lymphoma. Oncotarget 7, 86433–86445 (2016).
Acknowledgements
K.H.Y. acknowledges support from the US NIH National Cancer Institute (grants R01CA138688, R01CA187415 and 1RC1CA146299), The University of Texas MD Anderson Cancer Center Institutional Research and Development Fund, the Gundersen Lutheran Medical Foundation, the Hagemeister Lymphoma Foundation and the University Cancer Foundation via the Sister Institution Network Fund at The University of Texas MD Anderson Cancer Center. The work of the authors is also partially supported by NIH National Cancer Institute grants P50CA136411 and P50CA142509 and by the MD Anderson Cancer Center Support Grant CA016672.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
K.H.Y. has received research support from Adaptive Biotechnology, Dai Sanyo, Gilead Sciences, HTG Molecular Diagnostics, Incyte Pharmaceutical, Roche Molecular Systems and Seattle Genetics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Miao, Y., Medeiros, L.J., Li, Y. et al. Genetic alterations and their clinical implications in DLBCL. Nat Rev Clin Oncol 16, 634–652 (2019). https://doi.org/10.1038/s41571-019-0225-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-019-0225-1
This article is cited by
-
Mutation landscape in Chinese nodal diffuse large B-cell lymphoma by targeted next generation sequencing and their relationship with clinicopathological characteristics
BMC Medical Genomics (2024)
-
Molecular complexity of diffuse large B-cell lymphoma: a molecular perspective and therapeutic implications
Journal of Applied Genetics (2024)
-
Accurate interpretation of p53 immunohistochemical patterns is a surrogate biomarker for TP53 alterations in large B-cell lymphoma
BMC Cancer (2023)
-
Histone regulator KAT2A acts as a potential biomarker related to tumor microenvironment and prognosis of diffuse large B cell lymphoma
BMC Cancer (2023)
-
Novel roles of PIWI proteins and PIWI-interacting RNAs in human health and diseases
Cell Communication and Signaling (2023)