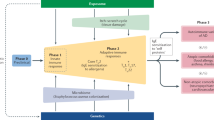
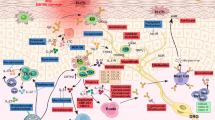
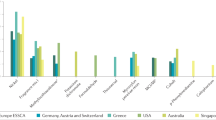
Abstract
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, with a lifetime prevalence of up to 20% and substantial effects on quality of life. AD is characterized by intense itch, recurrent eczematous lesions and a fluctuating course. AD has a strong heritability component and is closely related to and commonly co-occurs with other atopic diseases (such as asthma and allergic rhinitis). Several pathophysiological mechanisms contribute to AD aetiology and clinical manifestations. Impairment of epidermal barrier function, for example, owing to deficiency in the structural protein filaggrin, can promote inflammation and T cell infiltration. The immune response in AD is skewed towards T helper 2 cell-mediated pathways and can in turn favour epidermal barrier disruption. Other contributing factors to AD onset include dysbiosis of the skin microbiota (in particular overgrowth of Staphylococcus aureus), systemic immune responses (including immunoglobulin E (IgE)-mediated sensitization) and neuroinflammation, which is involved in itch. Current treatments for AD include topical moisturizers and anti-inflammatory agents (such as corticosteroids, calcineurin inhibitors and cAMP-specific 3ʹ,5ʹ-cyclic phosphodiesterase 4 (PDE4) inhibitors), phototherapy and systemic immunosuppressants. Translational research has fostered the development of targeted small molecules and biologic therapies, especially for moderate-to-severe disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Deckers, I. A. et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS ONE 7, e39803 (2012).
Dalgard, F. J. et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Invest. Dermatol. 135, 984–991 (2015).
Schmitt, J. et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J. Invest. Dermatol. 135, 24–30 (2015). This paper describes the roadmap guiding the process of development and implementation of core outcome sets for AD developed by the global multi-stakeholder Harmonizing Outcomes Measures for Eczema initiative.
Abuabara, K., Yu, A. M., Okhovat, J. P., Allen, E. & Langan, S. M. The prevalence of atopic dermatitis beyond childhood: A systematic review and meta-analysis of longitudinal studies. Allergy 73, 696–704 (2017).
Silverberg, J. I. & Hanifin, J. M. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J. Allergy Clin. Immunol. 132, 1132–1138 (2013).
Hay, R. J. et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J. Invest. Dermatol. 134, 1527–1534 (2014).
Perkin, M. R., Strachan, D. P., Williams, H. C., Kennedy, C. T. & Golding, J. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr. Allergy Immunol. 15, 221–229 (2004).
Garmhausen, D. et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 68, 498–506 (2013).
Margolis, J. S., Abuabara, K., Bilker, W., Hoffstad, O. & Margolis, D. J. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 150, 593–600 (2014).
Asher, M. I. et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 368, 733–743 (2006).
Williams, H., Stewart, A., von Mutius, E., Cookson, W. & Anderson, H. R. Is eczema really on the increase worldwide? J. Allergy Clin. Immunol. 121, 947–954 e15 (2008).
Apfelbacher, C. J., Diepgen, T. L. & Schmitt, J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy 66, 206–213 (2011).
Wadonda-Kabondo, N. et al. Association of parental eczema, hayfever, and asthma with atopic dermatitis in infancy: birth cohort study. Arch. Dis. Child 89, 917–921 (2004).
Flohr, C. & Mann, J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 69, 3–16 (2014).
Kantor, R. & Silverberg, J. I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 13, 15–26 (2017).
Bloomfield, S. F. et al. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect. Public Health 136, 213–224 (2016).
Flohr, C., Johansson, S. G., Wahlgren, C. F. & Williams, H. How atopic is atopic dermatitis? J. Allergy Clin. Immunol. 114, 150–158 (2004).
Manam, S., Tsakok, T., Till, S. & Flohr, C. The association between atopic dermatitis and food allergy in adults. Curr. Opin. Allergy Clin. Immunol. 14, 423–429 (2014).
Bergmann, M. M., Caubet, J. C., Boguniewicz, M. & Eigenmann, P. A. Evaluation of food allergy in patients with atopic dermatitis. J. Allergy Clin. Immunol. Pract. 1, 22–28 (2013).
Longo, G., Berti, I., Burks, A. W., Krauss, B. & Barbi, E. IgE-mediated food allergy in children. Lancet 382, 1656–1664 (2013).
Werfel, T. et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J. Allergy Clin. Immunol. 136, 96–103 (2015).
van der Hulst, A. E., Klip, H. & Brand, P. L. Risk of developing asthma in young children with atopic eczema: a systematic review. J. Allergy Clin. Immunol. 120, 565–569 (2007).
Kapoor, R. et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J. Am. Acad. Dermatol. 58, 68–73 (2008).
Pinart, M. et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir. Med. 2, 131–140 (2014).
Ferreira, M. A. et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat. Genet. 49, 1752–1757 (2017).
Yaghmaie, P., Koudelka, C. W. & Simpson, E. L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 131, 428–433 (2013).
Schmitt, J., Buske-Kirschbaum, A. & Roessner, V. Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review. Allergy 65, 1506–1524 (2010).
Thyssen, J. P. et al. Atopic dermatitis is associated with anxiety, depression, and suicidal ideation, but not with psychiatric hospitalization or suicide. Allergy 73, 214–220 (2017).
Yu, S. H. & Silverberg, J. I. Association between atopic dermatitis and depression in US adults. J. Invest. Dermatol. 135, 3183–3186 (2015).
Schmitt, J. et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J. Allergy Clin. Immunol. 137, 130–136 (2016).
Andersen, Y. M., Egeberg, A., Gislason, G. H., Skov, L. & Thyssen, J. P. Autoimmune diseases in adults with atopic dermatitis. J. Am. Acad. Dermatol. 76, 274–280 e1 (2017).
Drucker, A. M. et al. Incident alopecia areata and vitiligo in adult women with atopic dermatitis: nurses’ health study 2. Allergy 72, 831–834 (2017).
Sorrell, J., Petukhova, L., Reingold, R., Christiano, A. & Garzon, M. Shedding light on alopecia areata in pediatrics: a retrospective analysis of comorbidities in children in the national alopecia areata registry. Pediatr. Dermatol. 34, e271–e272 (2017).
Legendre, L. et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 72, 992–1002 (2015).
Kumar, P. & Subramaniyam, G. Molecular underpinnings of Th17 immune-regulation and their implications in autoimmune diabetes. Cytokine 71, 366–376 (2015).
Drucker, A. M., Qureshi, A. A., Dummer, T. J. B., Parker, L. & Li, W. Q. Atopic dermatitis and risk of hypertension, type 2 diabetes, myocardial infarction and stroke in a cross-sectional analysis from the Canadian partnership for tomorrow project. Br. J. Dermatol. 177, 1043–1051 (2017).
Standl, M. et al. Association of atopic dermatitis with cardiovascular risk factors and diseases. J. Invest. Dermatol. 137, 1074–1081 (2017).
Egeberg, A., Andersen, Y. M., Gislason, G. H., Skov, L. & Thyssen, J. P. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy 72, 783–791 (2017).
Silverwood, R. J. et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ 361, k1786 (2018).
Elias, P. M. & Steinhoff, M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J. Invest. Dermatol. 128, 1067–1070 (2008).
Thomsen, S. F. et al. Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy Asthma Proc. 28, 535–539 (2007).
Ellinghaus, D. et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat. Genet. 45, 808–812 (2013).
Esparza-Gordillo, J. et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat. Genet. 41, 596–601 (2009).
Paternoster, L. et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat. Genet. 44, 187–192 (2012).
Sun, L. D. et al. Genome-wide association study identifies two new susceptibility loci for atopic dermatitis in the Chinese Han population. Nat. Genet. 43, 690–694 (2011).
Hirota, T. et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat. Genet. 44, 1222–1226 (2012).
Paternoster, L. et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 47, 1449–1456 (2015). This is the largest genetic study of AD to date and provides a comprehensive view on AD genetic architecture and its overlap with other inflammatory diseases.
Schaarschmidt, H. et al. A genome-wide association study reveals 2 new susceptibility loci for atopic dermatitis. J. Allergy Clin. Immunol. 136, 802–806 (2015).
Irvine, A. D., McLean, W. H. & Leung, D. Y. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 365, 1315–1327 (2011). This review describes our current understanding of the manifold effects of an inherited filaggrin deficiency on skin barrier function and risk of AD and other atopic processes.
Baurecht, H. et al. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J. Allergy Clin. Immunol. 120, 1406–1412 (2007).
Weidinger, S. et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 121, 1203–1209 (2008).
Elias, M. S. et al. Proteomic analysis of filaggrin deficiency identifies molecular signatures characteristic of atopic eczema. J. Allergy Clin. Immunol. 140, 1299–1309 (2017).
Kawasaki, H. et al. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J. Allergy Clin. Immunol. 129, 1538–1546 (2012).
Scharschmidt, T. C. et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J. Allergy Clin. Immunol. 124, 496–506 (2009).
Brown, S. J. et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J. Invest. Dermatol. 132, 98–104 (2012).
Weidinger, S. et al. A genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum. Mol. Genet. 22, 4841–4856 (2013).
Paternoster, L. et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat. Genet. 44, 187–192 (2011).
Koh, B. H. et al. Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma. Proc. Natl Acad. Sci. USA 107, 10614–10619 (2010).
Kretschmer, A. et al. A common atopy-associated variant in the Th2 cytokine locus control region impacts transcriptional regulation and alters SMAD3 and SP1 binding. Allergy 69, 632–642 (2014).
Manz, J. et al. Targeted resequencing and functional testing identifies low-frequency missense variants in the gene encoding GARP as significant contributors to atopic dermatitis risk. J. Invest. Dermatol. 136, 2380–2386 (2016).
Cole, C. et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J. Allergy Clin. Immunol. 134, 82–91 (2014).
Seltmann, J., Roesner, L. M., von Hesler, F. W., Wittmann, M. & Werfel, T. IL-33 impacts on the skin barrier by downregulating the expression of filaggrin. J. Allergy Clin. Immunol. 135, 1659–1661 (2015).
Jungersted, J. M. et al. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy 65, 911–918 (2010).
Flohr, C. et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br. J. Dermatol. 163, 1333–1336 (2010).
Danby, S. G. et al. The effect of water hardness on surfactant deposition following washing and subsequent skin irritation in atopic dermatitis patients and healthy controls. J. Invest. Dermatol. 138, 68–77 (2017).
Halling-Overgaard, A. S. et al. Skin absorption through atopic dermatitis skin: a systematic review. Br. J. Dermatol. 177, 84–106 (2017).
Gao, P. S. et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J. Allergy Clin. Immunol. 124, 507–513 (2009).
Miajlovic, H., Fallon, P. G., Irvine, A. D. & Foster, T. J. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J. Allergy Clin. Immunol. 126, 1184–1190 e3 (2010).
Pellerin, L. et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J. Allergy Clin. Immunol. 131, 1094–1102 (2013).
Igawa, S. et al. Incomplete KLK7 Secretion and upregulated LEKTI expression underlie hyperkeratotic stratum corneum in atopic dermatitis. J. Invest. Dermatol. 137, 449–456 (2017).
Rawlings, A. V. & Voegeli, R. Stratum corneum proteases and dry skin conditions. Cell Tissue Res. 351, 217–235 (2013).
Ishikawa, J. et al. Changes in the ceramide profile of atopic dermatitis patients. J. Invest. Dermatol. 130, 2511–2514 (2010).
Janssens, M. et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 53, 2755–2766 (2012).
De Benedetto, A. et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 127, 773–786 e1-7 (2011).
Yoshida, K. et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J. Allergy Clin. Immunol. 134, 856–864 (2014). This study demonstrates that an increased number of Langerhans cells localize closely below the skin surface in AD lesions and elongate dendrites that penetrate tight junctions and can potentially take up protein antigens independent of IgE-based mechanisms.
Kong, H. H. et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 22, 850–859 (2012). This is the first study in which the skin microbiome of individuals with AD is longitudinally sampled and sequenced.
Oyoshi, M. K., Larson, R. P., Ziegler, S. F. & Geha, R. S. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 126, 976–984 (2010).
Yoon, J. et al. IL-23 induced in keratinocytes by endogenous TLR4 ligands polarizes dendritic cells to drive IL-22 responses to skin immunization. J. Exp. Med. 213, 2147–2166 (2016).
Kezic, S. et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J. Allergy Clin. Immunol. 129, 1031–1039 e1 (2012).
Fallon, P. G. et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat. Genet. 41, 602–608 (2009).
Omori-Miyake, M., Yamashita, M., Tsunemi, Y., Kawashima, M. & Yagi, J. In vitro assessment of IL-4- or IL-13-mediated changes in the structural components of keratinocytes in mice and humans. J. Invest. Dermatol. 134, 1342–1350 (2014).
Engelhardt, K. R. et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J. Allergy Clin. Immunol. 136, 402–412 (2015).
Yamamura, K. et al. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat. Commun. 8, 13946 (2017).
Murota, H. et al. Artemin causes hypersensitivity to warm sensation, mimicking warmth-provoked pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 130, 671–682 e4 (2012).
Gittler, J. K. et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 130, 1344–1354 (2012).
Suarez-Farinas, M. et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J. Allergy Clin. Immunol. 127, 954–964 (2011). This study finds that non-lesional skin of patients with AD already shows signs of subclinical inflammation and disturbed epidermal differentiation.
Noda, S. et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 136, 1254–1264 (2015).
Gandhi, N. A. et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 15, 35–50 (2016).
Virtanen, T. et al. No relationship between skin-infiltrating TH2-like cells and allergen-specific IgE response in atopic dermatitis. J. Allergy Clin. Immunol. 96, 411–420 (1995).
Tang, T. S., Bieber, T. & Williams, H. C. Does “autoreactivity” play a role in atopic dermatitis? J. Allergy Clin. Immunol. 129, 1209–1215 (2012).
Sonesson, A. et al. Sensitization to skin-associated microorganisms in adult patients with atopic dermatitis is of importance for disease severity. Acta Derm. Venereol. 93, 340–345 (2013).
van Reijsen, F. C., Bruijnzeel-Koomen, C. A., de Weger, R. A. & Mudde, G. C. Retention of long-lived, allergen-specific T cells in atopic dermatitis skin -letter. J. Invest. Dermatol. 108, 530 (1997).
Heratizadeh, A. et al. The role of T cell reactivity towards the autoantigen alpha-NAC in atopic dermatitis. Br. J. Dermatol. 164, 316–324 (2011).
Roesner, L. M. et al. alpha-NAC-specific autoreactive CD8+ T Cells in atopic dermatitis are of an effector memory type and secrete IL-4 and IFN-gamma. J. Immunol. 196, 3245–3252 (2016).
Brunner, P. M. et al. Nonlesional atopic dermatitis skin shares similar T cell clones with lesional tissues. Allergy 72, 2017–2025 (2017).
Bautista, D. M., Wilson, S. R. & Hoon, M. A. Why we scratch an itch: the molecules, cells and circuits of itch. Nat. Neurosci. 17, 175–182 (2014).
Feld, M. et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 138, 500–508 (2016).
Oh, M. H. et al. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol. 191, 5371–5382 (2013).
Kabashima, K., Otsuka, A. & Nomura, T. Linking air pollution to atopic dermatitis. Nat. Immunol. 18, 5–6 (2016).
Sanders, K. M. & Akiyama, T. The vicious cycle of itch and anxiety. Neurosci. Biobehav Rev. 87, 17–26 (2018).
Wilson, S. R. et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155, 285–295 (2013).
Chen, Y. et al. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J. Biol. Chem. 291, 10252–10262 (2016).
Oetjen, L. K. et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 171, 217–228 e13 (2017).
Beck, L. A. et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 371, 130–139 (2014). This is the first published phase III trial demonstrating the superiority of dupilumab over placebo in improving clinical scores in adults with moderate-to-severe AD.
Simpson, E. L., Akinlade, B. & Ardeleanu, M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 376, 1090–1091 (2017).
Cevikbas, F. et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133, 448–460 (2014). This study contains the results from a phase II trial demonstrating a significant effect of the anti-IL-31 antibody nemolizumab on pruritus in patients with moderate-to-severe AD.
Ruzicka, T. et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N. Engl. J. Med. 376, 826–835 (2017).
Tauber, M. et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J. Allergy Clin. Immunol. 137, 1272–1274 e3 (2016).
Totte, J. E. et al. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br. J. Dermatol. 175, 687–695 (2016).
Baurecht, H. et al. Epidermal lipid composition, barrier integrity and eczematous inflammation are associated with skin microbiome configuration. J. Allergy Clin. Immunol. 141, 1668–1676 (2018). This study demonstrates that epidermal barrier function and cutaneous inflammation shape the skin microbiota and that AD shows an altered microbial configuration across diverse body sites.
Byrd, A. L. et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 9, eaal4651 (2017).
Kennedy, E. A. et al. Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 139, 166–172 (2017). This study presents evidence from a prospective clinical trial that shows that colonization with non- S. aureus Staphylococcus spp. reduces the risk of AD in children.
Geoghegan, J. A., Irvine, A. D. & Foster, T. J. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. https://doi.org/10.1016/j.tim.2017.11.008 (2017).
Hiragun, T. et al. Fungal protein MGL_1304 in sweat is an allergen for atopic dermatitis patients. J. Allergy Clin. Immunol. 132, 608–615 (2013).
Weidinger, S. & Novak, N. Atopic dermatitis. Lancet 387, 1109–1122 (2016). This is a concise and comprehensive summary of the understanding of AD pathophysiology and clinical and epidemiological feature and of therapies that are used to treat the disease.
Eichenfield, L. F., Hanifin, J. M., Luger, T. A., Stevens, S. R. & Pride, H. B. Consensus conference on pediatric atopic dermatitis. J. Am. Acad. Dermatol. 49, 1088–1095 (2003).
Torrelo, A. Atopic dermatitis in different skin types. What is to know? J. Eur. Acad. Dermatol. Venereol. 28 (Suppl. 3), 2–4 (2014).
Chopra, R. et al. Severity strata for EASI, mEASI, oSCORAD, SCORAD, ADSI and BSA in adolescents and adults with atopic dermatitis. Br. J. Dermatol. 177, 1316–1321 (2017).
Leshem, Y. A., Hajar, T., Hanifin, J. M. & Simpson, E. L. What the eczema area and severity index score tells us about the severity of atopic dermatitis: an interpretability study. Br. J. Dermatol. 172, 1353–1357 (2015).
Schmitt, J., Langan, S. & Williams, H. C. What are the best outcome measurements for atopic eczema? A systematic review. J. Allergy Clin. Immunol. 120, 1389–1398 (2007).
Spuls, P. I. et al. The International TREatment of ATopic Eczema (TREAT) registry taskforce: an initiative to harmonize data collection across national atopic eczema photo- and systemic therapy registries. J. Invest. Dermatol. 137, 2014–2016 (2017).
Mohan, G. C. & Lio, P. A. Comparison of dermatology and allergy guidelines for atopic dermatitis management. JAMA Dermatol. 151, 1009–1013 (2015).
Kim, Y. M. et al. Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea. PLoS ONE 12, e0175229 (2017).
Roerdink, E. M. et al. Association of food allergy and atopic dermatitis exacerbations. Ann. Allergy Asthma Immunol. 116, 334–338 (2016).
Darsow, U. et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy 59, 1318–1325 (2004).
Werfel, T. et al. Approach to suspected food allergy in atopic dermatitis. Guideline of the task force on food allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J. Dtsch. Dermatol. Ges. 7, 265–271 (2009).
Wollenberg, A. et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 30, 729–747 (2016).
Sinagra, J. L. et al. Unnecessary milk elimination diets in children with atopic dermatitis. Pediatr. Dermatol. 24, 1–6 (2007).
Du Toit, G. et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 372, 803–813 (2015). This study presents results from the landmark Learning Early about Peanut Allergy (LEAP) trial demonstrating that early ingestion of peanut protein in infants at high risk of developing peanut allergy dramatically reduces the development of such allergy.
Tam, H. H. et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy 71, 1345–1356 (2016).
Bath-Hextall, F. J., Jenkinson, C., Humphreys, R. & Williams, H. C. Dietary supplements for established atopic eczema. Cochrane Database Syst. Rev. 2, CD005205 (2012).
Host, A. et al. Dietary prevention of allergic diseases in infants and small children. Pediatr. Allergy Immunol. 19, 1–4 (2008).
Pelucchi, C. et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 23, 402–414 (2012).
Van Bever, H. P., Nagarajan, S., Shek, L. P. & Lee, B. W. OPINION: primary prevention of allergy - will it soon become a reality? Pediatr. Allergy Immunol. 27, 6–12 (2016).
Feeney, M. et al. Impact of peanut consumption in the LEAP Study: feasibility, growth, and nutrition. J. Allergy Clin. Immunol. 138, 1108–1118 (2016).
Roduit, C. et al. Development of atopic dermatitis according to age of onset and association with early-life exposures. J. Allergy Clin. Immunol. 130, 130–136 (2012).
Simpson, E. L. et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 134, 818–823 (2014). This study presents results from two randomized controlled pilot studies indicating that regular emollient application from birth might have preventative effects on AD development.
Horimukai, K. et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J. Allergy Clin. Immunol. 134, 824–830 (2014).
Heratizadeh, A. et al. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J. Allergy Clin. Immunol. 140, 845–853 (2017).
Pustisek, N. et al. The significance of structured parental educational intervention on childhood atopic dermatitis: a randomized controlled trial. J. Eur. Acad. Dermatol. Venereol. 30, 806–812 (2016).
Pantazi, E., Valenza, G., Hess, M. & Hamad, B. The atopic dermatitis market. Nat. Rev. Drug Discov. 17, 237–238 (2018).
Cork, M. J. et al. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. Br. J. Dermatol. 149, 582–589 (2003).
van Zuuren, E. J., Fedorowicz, Z. & Arents, B. W. M. Emollients and moisturizers for eczema: abridged Cochrane systematic review including GRADE assessments. Br. J. Dermatol. 177, 1256–1271 (2017).
Brunner, P. M. et al. A mild topical steroid leads to progressive anti-inflammatory effects in the skin of patients with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 138, 169–178 (2016).
Danby, S. G., Chittock, J., Brown, K., Albenali, L. H. & Cork, M. J. The effect of tacrolimus compared with betamethasone valerate on the skin barrier in volunteers with quiescent atopic dermatitis. Br. J. Dermatol. 170, 914–921 (2014).
Weidinger, S., Baurecht, H. & Schmitt, J. A. 5-year randomized trial on the safety and efficacy of pimecrolimus in atopic dermatitis: a critical appraisal. Br. J. Dermatol. 177, 999–1003 (2017).
Castela, E. et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J. Eur. Acad. Dermatol. Venereol. 26 (Suppl. 3), 47–51 (2012).
Nakahara, T., Morimoto, H., Murakami, N. & Furue, M. Mechanistic insights into topical tacrolimus for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 29, 233–238 (2017).
Jensen, J. M. et al. Effects of pimecrolimus compared with triamcinolone acetonide cream on skin barrier structure in atopic dermatitis: a randomized, double-blind, right-left arm trial. Acta Derm. Venereol. 93, 515–519 (2013).
Jensen, J. M. et al. Gene expression is differently affected by pimecrolimus and betamethasone in lesional skin of atopic dermatitis. Allergy 67, 413–423 (2012).
Ashcroft, D. M., Dimmock, P., Garside, R., Stein, K. & Williams, H. C. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ 330, 516 (2005).
Zebda, R. & Paller, A. S. Phosphodiesterase 4 inhibitors. J. Am. Acad. Dermatol. 78, S43–S52 (2018).
Eichenfield, L. F. et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J. Am. Acad. Dermatol. 77, 641–649 (2017).
Ahmed, A., Solman, L. & Williams, H. C. Magnitude of benefit for topical crisaborole in the treatment of atopic dermatitis in children and adults does not look promising: a critical appraisal. Br. J. Dermatol. 178, 659–662 (2017).
Garritsen, F. M., Brouwer, M. W., Limpens, J. & Spuls, P. I. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br. J. Dermatol. 170, 501–513 (2014).
Rodenbeck, D. L., Silverberg, J. I. & Silverberg, N. B. Phototherapy for atopic dermatitis. Clin. Dermatol. 34, 607–613 (2016).
Simpson, E. L. et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J. Am. Acad. Dermatol. 77, 623–633 (2017).
Schmitt, J., Schmitt, N. & Meurer, M. Cyclosporin in the treatment of patients with atopic eczema - a systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 21, 606–619 (2007).
Zachariae, H., Kragballe, K., Hansen, H. E., Marcussen, N. & Olsen, S. Renal biopsy findings in long-term cyclosporin treatment of psoriasis. Br. J. Dermatol. 136, 531–535 (1997).
Eichenfield, L. F. et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J. Allergy Clin. Immunol. 139, S49–S57 (2017).
Flohr, C. & Irvine, A. D. Systemic therapies for severe atopic dermatitis in children and adults. J. Allergy Clin. Immunol. 132, 774–774 (2013).
Dvorakova, V., O’Regan, G. M. & Irvine, A. D. Methotrexate for severe childhood atopic dermatitis: clinical experience in a tertiary center. Pediatr. Dermatol. 34, 528–534 (2017).
Fuggle, N. R. et al. The adverse effect profile of oral azathioprine in pediatric atopic dermatitis, and recommendations for monitoring. J. Am. Acad. Dermatol. 72, 108–114 (2015).
Goujon, C. et al. Methotrexate versus cyclosporine in adults with moderate-to-severe atopic dermatitis: a phase III randomized noninferiority trial. J. Allergy Clin. Immunol. Pract. 6, 562–569 (2017).
Schram, M. E. et al. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J. Allergy Clin. Immunol. 128, 353–359 (2011).
Roekevisch, E. et al. Methotrexate versus azathioprine in patients with atopic dermatitis: two years follow-up data. J. Allergy Clin. Immunol. 141, 825–827 (2017).
Haeck, I. M. et al. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J. Am. Acad. Dermatol. 64, 1074–1084 (2011).
van der Schaft, J. et al. Drug survival for ciclosporin A in a long-term daily practice cohort of adult patients with atopic dermatitis. Br. J. Dermatol. 172, 1621–1627 (2015).
van der Schaft, J. et al. Drug survival for azathioprine and enteric-coated mycophenolate sodium in a long-term daily practice cohort of adult patients with atopic dermatitis. Br. J. Dermatol. 175, 199–202 (2016).
Drucker, A. M. et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br. J. Dermatol 178, 768–775 (2018).
Simpson, E. L. et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 375, 2335–2348 (2016).
Blauvelt, A. et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 389, 2287–2303 (2017).
Paternoster, L. et al. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J. Allergy Clin. Immunol. 141, 964–971 (2018). This is a large cohort study providing evidence for distinct subphenotypes of AD in children on the basis of temporal trajectories and genetic markers.
Skabytska, Y., Kaesler, S., Volz, T. & Biedermann, T. The role of innate immune signaling in the pathogenesis of atopic dermatitis and consequences for treatments. Semin. Immunopathol. 38, 29–43 (2016).
Kong, H. H. & Segre, J. A. Skin microbiome: looking back to move forward. J. Invest. Dermatol. 132, 933–939 (2012).
Park, H. Y. et al. Staphylococcus aureus colonization in acute and chronic skin lesions of patients with atopic dermatitis. Ann. Dermatol. 25, 410–416 (2013).
Beck, L. A. et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J. Allergy Clin. Immunol. 124, 260–269 (2009).
Hinz, T. et al. Atopic dermo-respiratory syndrome is a correlate of eczema herpeticum. Allergy 66, 925–933 (2011).
Olsen, J. R., Gallacher, J., Piguet, V. & Francis, N. A. Epidemiology of molluscum contagiosum in children: a systematic review. Fam. Pract. 31, 130–136 (2014).
Wollenberg, A., Wetzel, S., Burgdorf, W. H. & Haas, J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J. Allergy Clin. Immunol. 112, 667–674 (2003).
Mathes, E. F. et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics 132, e149–e157 (2013).
Oldhoff, J. M. et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 60, 693–696 (2005).
Khattri, S. et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp. Dermatol. 26, 28–35 (2017).
Saeki, H. et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br. J. Dermatol. 177, 419–427 (2017).
Basra, M. K., Fenech, R., Gatt, R. M., Salek, M. S. & Finlay, A. Y. The dermatology life quality index 1994–2007: a comprehensive review of validation data and clinical results. Br. J. Dermatol. 159, 997–1035 (2008).
Karimkhani, C. et al. Global skin disease morbidity and mortality: an update from the global burden of disease study 2013. JAMA Dermatol. 153, 406–412 (2017).
Heinl, D. et al. Measurement properties of quality-of-life measurement instruments for infants, children and adolescents with eczema: a systematic review. Br. J. Dermatol. 176, 878–889 (2017).
Hill, M. K., Kheirandish Pishkenari, A., Braunberger, T. L., Armstrong, A. W. & Dunnick, C. A. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J. Am. Acad. Dermatol. 75, 906–917 (2016).
Beattie, P. E. & Lewis-Jones, M. S. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br. J. Dermatol. 155, 145–151 (2006).
Drucker, A. M. et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J. Invest. Dermatol. 137, 26–30 (2017).
Holm, J. G., Agner, T., Clausen, M. L. & Thomsen, S. F. Quality of life and disease severity in patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 30, 1760–1767 (2016).
Anderson, R. T. & Rajagopalan, R. Effects of allergic dermatosis on health-related quality of life. Curr. Allergy Asthma Rep. 1, 309–315 (2001).
Beikert, F. C. et al. Willingness to pay and quality of life in patients with atopic dermatitis. Arch. Dermatol. Res. 306, 279–286 (2014).
Eckert, L. et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J. Am. Acad. Dermatol. 77, 274–279 (2017).
Wang, I. J., Wang, J. Y. & Yeh, K. W. Childhood atopic dermatitis in Taiwan. Pediatr. Neonatol. 57, 89–96 (2016).
Heede, N. G. et al. Health-related quality of life in adult dermatitis patients stratified by filaggrin genotype. Contact Dermatitis 76, 167–177 (2017).
Simpson, E. L. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol. Ther. 7, 243–248 (2017).
Bieber, T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy 67, 1475–1482 (2012).
Dyjack, N. et al. Minimally invasive skin tape strip RNA sequencing identifies novel characteristics of the type 2-high atopic dermatitis disease endotype. J. Allergy Clin. Immunol. 141, 1298–1309 (2018).
Thijs, J. L. et al. Moving toward endotypes in atopic dermatitis: identification of patient clusters based on serum biomarker analysis. J. Allergy Clin. Immunol. 140, 730–737 (2017).
Schmitt, J. et al. Usage and effectiveness of systemic treatments in adults with severe atopic eczema: first results of the German Atopic Eczema Registry TREATgermany. J. Dtsch. Dermatol. Ges. 15, 49–59 (2017).
Anbunathan, H. & Bowcock, A. M. The molecular revolution in cutaneous biology: the era of genome-wide association studies and statistical, big data, and computational topics. J. Invest. Dermatol. 137, e113–e118 (2017).
Coca, A. & Cooke, R. l. On the classification of the phenomena of hypersensitiveness. J. Immunol. 8, 163–182 (1923).
Johansson, S. G. et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 113, 832–836 (2004).
Tsakok, T. et al. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 137, 1071–1078 (2016).
Kelleher, M. M. et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. J. Allergy Clin. Immunol. 137, 1111–1116 e8 (2016).
Flohr, C. et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J. Invest. Dermatol. 134, 345–350 (2014).
Brough, H. A. et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J. Allergy Clin. Immunol. 135, 164–170 (2015).
Heil, P. M., Maurer, D., Klein, B., Hultsch, T. & Stingl, G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. J. Dtsch. Dermatol. Ges. 8, 990–998 (2010).
van Zuuren, E. J. et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst. Rev. 3, 25 (2014).
Illi, S. et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J. Allergy Clin. Immunol. 113, 925–931 (2004).
Belgrave, D. C. et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med. 11, e1001748 (2014).
Renz, H. et al. Food allergy. Nat. Rev. Dis. Primers 4, 17098 (2018).
du Toit, G. et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the learning early about peanut allergy study cohort. J. Allergy Clin. Immunol. 141, 1343–1353 (2018).
Brown, S. J. et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J. Allergy Clin. Immunol. 127, 661–667 (2011).
Onoue, A., Kabashima, K., Kobayashi, M., Mori, T. & Tokura, Y. Induction of eosinophil- and Th2-attracting epidermal chemokines and cutaneous late-phase reaction in tape-stripped skin. Exp. Dermatol. 18, 1036–1043 (2009).
Salimi, M. et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 210, 2939–2950 (2013).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3, 673–680 (2002). This is the first published report demonstrating high TSLP expression in AD, potentiation of inflammatory T H 2 responses by TSLP and a potential role for TSLP in airway allergic disease.
Ito, T. et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202, 1213–1223 (2005).
Hijnen, D. et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. J. Invest. Dermatol. 133, 973–979 (2013).
Mashiko, S., Mehta, H., Bissonnette, R. & Sarfati, M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J. Dermatol. Sci. 88, 167–174 (2017).
Bruggen, M. C. et al. In situ mapping of innate lymphoid cells in human skin: evidence for remarkable differences between normal and inflamed skin. J. Invest. Dermatol. 136, 2396–2405 (2016).
Maggi, L. et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J. Allergy Clin. Immunol. 139, 964–976 (2017).
Islam, S. A. & Luster, A. D. T cell homing to epithelial barriers in allergic disease. Nat. Med. 18, 705–715 (2012).
Watanabe, R. et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 7, 279ra39 (2015).
Novak, N. An update on the role of human dendritic cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 129, 879–886 (2012).
Liu, B. et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl Acad. Sci. USA 113, E7572–E7579 (2016).
Paller, A. S., Kabashima, K. & Bieber, T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 140, 633–643 (2017).
Wollenberg, A. et al. A phase 2b dose-ranging efficacy and safety study of tralokinumab in adult patients with moderate to severe atopic dermatitis (AD) (Poster). SKIN The Journal of Cutaneous Medicine 2 (2017).
Simpson, E. L. et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 78, 863–871 (2018).
Taylor, N. P. After asthma success, AstraZeneca and Amgen’s tezepelumab misses in atopic dermatitis. FierceBiotech https://www.fiercebiotech.com/biotech/after-asthma-success-astrazeneca-and-amgen-s-tezepelumab-misses-atopic-dermatitis?utm_source=internal&utm_medium=rss (2017).
Londei, M. et al. A phase 1 study of ANB020, an anti-IL-33 monoclonal antibody, in healthy volunteers. J. Am. Acad. Dermatol. 76 (Suppl. 1), AB20 (2017).
Glenmark Pharmaceuticals. Glenmark Pharmaceuticals reports positive data in a phase 2a study of GBR 830 for the treatment of patients with atopic dermatitis. CISION https://www.prnewswire.com/news-releases/glenmark-pharmaceuticals-reports-positive-data-in-a-phase-2a-study-of-gbr-830-for-the-treatment-of-patients-with-atopic-dermatitis-300496604.html (2017).
Guttman-Yassky, E. et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 78, 872–881 (2018).
Vanda Pharmaceuticals Inc. Vanda’s tradipitant improves itch and disease severity in patients with atopic dermatitis. CISION https://www.prnewswire.com/news-releases/vandas-tradipitant-improves-itch-and-disease-severity-in-patients-with-atopic-dermatitis-300519177.html (2017).
Guttman-Yassky, E. et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. https://doi.org/10.1016/j.jaad.2018.01.018 (2018).
Abbvie. AbbVie’s Upadacitinib (ABT-494) Meets Primary Endpoint in Phase 2b Study in Atopic Dermatitis. AbbVie Newsroom https://news.abbvie.com/news/abbvies-upadacitinib-abt-494-meets-primary-endpoint-in-phase-2b-study-in-atopic-dermatitis.htm (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02780167 (2018).
Guttman-Yassky, E. et al. ASN002 a dual oral inhibitor of JAK/SYK signaling improves clinical outcomes and associated cutaneous inflammation in moderate-to-severe atopic dermatitis patients. [abstract 559] International Investigative Dermatology meeting (2018).
Novartis. Ziarco Pharma Announces Positive Top Line Results from Phase 2a Study in Moderate to Severe Atopic Dermatitis with its Oral Lead Compound ZPL-389. Lundbeckfonden https://www.lundbeckfonden.com/en/ziarco-pharma-announces-positive-top-line-results-from-phase-2a-study-in-moderate-to-severe-atopic-dermatitis-with-its-oral-lead-compound-zpl-389/ (2016).
Bissonnette, R. et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br. J. Dermatol. 175, 902–911 (2016).
Nakagawa, H., Nemoto, O., Igarashi, A. & Nagata, T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. Br. J. Dermatol. 178, 424–432 (2018).
Bissonnette, R. et al. Systemic pharmacokinetics, safety, and preliminary efficacy of topical AhR agonist tapinarof: results of a phase 1 study. Clin. Pharmacol. Drug Dev. https://doi.org/10.1002/cpdd.439 (2018).
Reviewer information
Nature Reviews Disease Primers would like to thank M. Furue, S. Ständer, E. Weisshaar and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (A.D.I. and S.W.); Epidemiology (S.W.); Mechanisms/pathophysiology (all authors); Diagnosis, screening and prevention (T.B.); Management (L.A.B., T.B., A.D.I. and S.W.); Quality of life (L.A.B.); Outlook (A.D.I. and S.W.); Overview of Primer (A.D.I. and S.W.).
Corresponding authors
Ethics declarations
Competing interests
S.W. has received honoraria for invited lectures from and served on advisory boards for Galderma, Novartis, Pfizer, Regeneron and Sanofi and has received research funding from La Roche-Posay, Novartis, Pfizer and Sanofi. L.A.B. has received consulting fees from AbbVie, AnaptysBio, Boehringer Ingelheim, Celgene, GlaxoSmithKline, Lilly, Novan, Novartis, Realm Therapeutics, Regeneron and Sanofi-Genzyme; she is principal investigator for clinical trials for AbbVie, Realm Therapeutics and Regeneron and is a stock owner of Medtronic and Pfizer. T.B. has received honoraria for invited lectures from and served on consultant and/or advisory boards for AbbVie, Allmiral, AnaptysBio, Asana Biosciences, Celgene, Daiichi-Sankyo, Dermavant, Galapagos, Galderma, Glenmark, GlaxoSmithKline, Kymab, LEO Pharma, La Roche-Posay, Lilly, L’Oréal, Menlo, Novartis, Pfizer, Roche–Genentech and Sanofi-Genzyme; he was principal investigator for clinical trials with Galderma, LEO Pharma, Lilly, Menlo, Pfizer, Regeneron–Sanofi and Roche–Genentech. K.K. has received honoraria for invited lectures from and served on consultant and/or advisory boards for Chugai, Kyowa Hakko Kirin, LEO Pharma, Mitsubishi Tanabe, Maruho, Pola and Regeneron–Sanof; he was principal investigator for clinical trials with Japan Tobacco, Maruho and Regeneron–Sanofi. A.D.I. has received honoraria for invited lectures from and served on consultant and/or advisory boards for AbbVie, Galderma, Lilly, Novartis, Pfizer, Roche–Genentech and Sanofi-Genzyme; he was principal investigator for clinical trials with AbbVie and Regeneron–Sanofi.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: www.clinicaltrials.gov
European Medicines Agency: www.EMEA.eu
Global Health Data Exchange: http://ghdx.healthdata.org
US FDA: www.fda.gov
VisualDx: www.visualdx.com
Rights and permissions
About this article
Cite this article
Weidinger, S., Beck, L.A., Bieber, T. et al. Atopic dermatitis. Nat Rev Dis Primers 4, 1 (2018). https://doi.org/10.1038/s41572-018-0001-z
Published:
DOI: https://doi.org/10.1038/s41572-018-0001-z
This article is cited by
-
Comparison of visual diagnostic accuracy of dermatologists practicing in Germany in patients with light skin and skin of color
Scientific Reports (2024)
-
Treat-to-Target in Atopic Dermatitis
American Journal of Clinical Dermatology (2024)
-
Cutaneous Components Leading to Pruritus, Pain, and Neurosensitivity in Atopic Dermatitis: A Narrative Review
Dermatology and Therapy (2024)
-
OX40 in the Pathogenesis of Atopic Dermatitis—A New Therapeutic Target
American Journal of Clinical Dermatology (2024)
-
Therapie-Update zur atopischen Dermatitis
hautnah (2024)