Abstract
The ability of growth hormone (GH) to induce adipose tissue lipolysis has been known for over five decades; however, the molecular mechanisms that mediate this effect and the ability of GH to inhibit insulin-stimulated glucose uptake have scarcely been documented. In this same time frame, our understanding of adipose tissue has evolved to reveal a complex structure with distinct types of adipocyte, depot-specific differences, a biologically significant extracellular matrix and important endocrine properties mediated by adipokines. All these aforementioned features, in turn, can influence lipolysis. In this Review, we provide a historical and current overview of the lipolytic effect of GH in humans, mice and cultured cells. More globally, we explain lipolysis in terms of GH-induced intracellular signalling and its effect on obesity, insulin resistance and lipotoxicity. In this regard, findings that define molecular mechanisms by which GH induces lipolysis are described. Finally, data are presented for the differential effect of GH on specific adipose tissue depots and on distinct classes of metabolically active adipocytes. Together, these cellular, animal and human studies reveal novel cellular phenotypes and molecular pathways regulating the metabolic effects of GH on adipose tissue.
Key points
Growth hormone (GH) exposure in humans potently stimulates the release of free fatty acids from adipose tissue into the circulation after a lag phase of 1–2 hours and with a peak effect after 3–4 hours.
This GH-induced increase in circulating free fatty acids is causally linked to the antagonistic effects of GH on basal and insulin-stimulated glucose uptake.
Overexpression of FSP27 or exposure to a GH receptor antagonist, pegvisomant, can block the diabetogenic effects of GH.
GH-induced activation of the MEK–ERK pathway has a key role in PPARγ inactivation and FSP27 downregulation, thus increasing lipolysis and insulin resistance.
GH impacts adipose tissue in a depot-specific manner and influences other features of adipose tissue (for example, senescence, adipocyte subpopulations and fibrosis), all of which could influence lipolysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brockmann, G. A. & Bevova, M. R. Using mouse models to dissect the genetics of obesity. Trends. Genet. 18, 367–376 (2002).
Friedman, J. M. A war on obesity, not the obese. Science 299, 856–858 (2003).
Moller, N. & Jorgensen, J. O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 30, 152–177 (2009).
Luft, R., Ikkos, D., Gemzell, C. A. & Olivecrona, H. Effect of human growth hormone in hypophysectomised diabetic subjects. Lancet 1, 721–722 (1958).
Davidson, M. B. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr. Rev. 8, 115–131 (1987). This classic review provides a historical perspective and a thorough understanding of early studies on the role of GH on carbohydrate and lipid metabolism.
Rabinowitz, D. & Zierler, K. L. A metabolic regulating device based on the actions of human growth hormone and of insulin, singly and together, on the human forearm. Nature 199, 913–915 (1963).
Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9, 352–355 (2003).
Nielsen, S., Moller, N., Christiansen, J. S. & Jorgensen, J. O. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 50, 2301–2308 (2001). The lipolytic action of GH is responsible for its reduction of insulin sensitivity in human volunteers.
Unger, R. H., Clark, G. O., Scherer, P. E. & Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta 1801, 209–214 (2010).
Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46, 3–10 (1997).
Boden, G., Chen, X., Ruiz, J., White, J. V. & Rossetti, L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J. Clin. Invest. 93, 2438–2446 (1994).
Dresner, A. et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103, 253–259 (1999).
Ferrannini, E., Barrett, E. J., Bevilacqua, S. & DeFronzo, R. A. Effect of fatty acids on glucose production and utilization in man. J. Clin. Invest. 72, 1737–1747 (1983).
Rajala, M. W. & Scherer, P. E. Minireview: the adipocyte — at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144, 3765–3773 (2003).
Ahmadian, M., Wang, Y. & Sul, H. S. Lipolysis in adipocytes. Int. J. Biochem. Cell Biol. 42, 555–559 (2010).
Jenkins, C. M. et al. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279, 48968–48975 (2004).
Villena, J. A., Roy, S., Sarkadi-Nagy, E., Kim, K. H. & Sul, H. S. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279, 47066–47075 (2004).
Zechner, R. et al. FAT SIGNALS — lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 (2012).
Zimmermann, R. et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004).
Granneman, J. G., Moore, H. P., Krishnamoorthy, R. & Rathod, M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (ABHD5) and adipose triglyceride lipase (ATGL). J. Biol. Chem. 284, 34538–34544 (2009).
Lass, A. et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 3, 309–319 (2006).
Subramanian, V. et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J. Biol. Chem. 279, 42062–42071 (2004).
Miyoshi, H. et al. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282, 996–1002 (2007).
Yang, X. et al. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 11, 194–205 (2010).
Gong, J. et al. FSP27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195, 953–963 (2011).
Keller, P. et al. Fat-specific protein 27 regulates storage of triacylglycerol. J. Biol. Chem. 283, 14355–14365 (2008).
Kim, J. Y. et al. Assessment of fat-specific protein 27 in the adipocyte lineage suggests a dual role for FSP27 in adipocyte metabolism and cell death. Am. J. Physiol. Endocrinol. Metab. 294, E654–E667 (2008).
Puri, V. et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 282, 34213–34218 (2007).
Nishino, N. et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118, 2808–2821 (2008).
Grahn, T. H. et al. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J. Biol. Chem. 289, 12029–12039 (2014).
Singh, M. et al. Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase. J. Biol. Chem. 289, 14481–14487 (2014).
Raben, M. S. Growth hormone. 1. Physiologic aspects. N. Engl. J. Med. 266, 31–35 (1962).
Raben, M. S. & Hollenberg, C. H. Effect of growth hormone on plasma fatty acids. J. Clin. Invest. 38, 484–488 (1959).
Glick, S. M., Roth, J., Yalow, R. S. & Berson, S. A. Immunoassay of human growth hormone in plasma. Nature 199, 784–787 (1963).
Giustina, A. & Veldhuis, J. D. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 19, 717–797 (1998).
Roth, J., Glick, S. M., Yalow, R. S. & Berson, S. A. Hypoglycemia: a potent stimulus to secretion of growth hormone. Science 140, 987–988 (1963).
Zierler, K. L. & Rabinowitz, D. Roles of insulin and growth hormone, based on studies of forearm metabolism in man. Medicine 42, 385–402 (1963).
Richelsen, B. et al. Growth hormone treatment of obese women for 5 wk: effect on body composition and adipose tissue LPL activity. Am. J. Physiol. 266, E211–E216 (1994).
Krag, M. B. et al. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am. J. Physiol. Endocrinol. Metab. 292, E920–E927 (2007).
Norrelund, H. et al. Effects of GH on urea, glucose and lipid metabolism, and insulin sensitivity during fasting in GH-deficient patients. Am. J. Physiol. Endocrinol. Metab. 285, E737–E743 (2003).
Jorgensen, J. O. et al. Marked effects of sustained low growth hormone (GH) levels on day-to-day fuel metabolism: studies in GH-deficient patients and healthy untreated subjects. J. Clin. Endocrinol. Metab. 77, 1589–1596 (1993).
Jorgensen, J. O. et al. Growth hormone versus placebo treatment for one year in growth hormone deficient adults: increase in exercise capacity and normalization of body composition. Clin. Endocrinol. 45, 681–688 (1996).
Moller, N. et al. Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J. Clin. Endocrinol. Metab. 74, 1012–1019 (1992).
Bredella, M. A. et al. Body composition and ectopic lipid changes with biochemical control of acromegaly. J. Clin. Endocrinol. Metab. 102, 4218–4225 (2017).
Krusenstjerna-Hafstrom, T. et al. Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J. Clin. Endocrinol. Metab. 96, 2548–2557 (2011).
Shulman, G. I. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology 19, 183–190 (2004).
Nielsen, C. et al. Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J. Clin. Endocrinol. Metab. 93, 2842–2850 (2008).
Jessen, N. et al. Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am. J. Physiol. Endocrinol. Metab. 288, E194–E199 (2005).
del Rincon, J. P. et al. Growth hormone regulation of p85α expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes 56, 1638–1646 (2007).
Dominici, F. P. et al. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm. IGF Res. 15, 324–336 (2005).
Nellemann, B. et al. Growth hormone-induced insulin resistance in human subjects involves reduced pyruvate dehydrogenase activity. Acta Physiol. 210, 392–402 (2014).
Mekala, K. C. & Tritos, N. A. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J. Clin. Endocrinol. Metab. 94, 130–137 (2009).
Thankamony, A. et al. Short-term administration of pegvisomant improves hepatic insulin sensitivity and reduces soleus muscle intramyocellular lipid content in young adults with type 1 diabetes. J. Clin. Endocrinol. Metab. 99, 639–647 (2014).
Sharma, R. et al. Growth hormone controls lipolysis by regulation of FSP27 expression. J. Endocrinol. 239, 289–301 (2018). In both mice and cell culture models, GH regulates lipolysis and insulin sensitivity employing ERK-dependent and STAT5-dependent mechanisms to control PPARγ-mediated transcription of FSP27.
Sharma, V. M. et al. Growth hormone acts along the PPARγ-FSP27 axis to stimulate lipolysis in human adipocytes. Am. J. Physiol. Endocrinol. Metab. 316, E34–E42 (2019). In human adipocytes, GH regulates lipolysis through ERK-dependent phosphorylation of PPARγ and transcriptional regulation of FSP27.
Troike, K. M. et al. Impact of growth hormone on regulation of adipose tissue. Compr. Physiol. 7, 819–840 (2017).
Brooks, A. J. & Waters, M. J. The growth hormone receptor: mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 6, 515–525 (2010). This excellent article provides a thorough understanding of the GH–GHR interaction as a function of downstream intracellular signalling.
Rowlinson, S. W. et al. An agonist-induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat. Cell Biol. 10, 740–747 (2008). The GH–GHR interaction can activate both STAT5 and ERK dependent intracellular signalling pathways. In light of findings that GH-lipolysis and insulin resistance are primarily dependent on ERK-dependent signaling, specific and yet to be discovered ERK-dependent GH analogues might have strong therapeutic and clinical significance.
Waters, M. J. The growth hormone receptor. Growth. Horm. IGF Res. 28, 6–10 (2016).
Lanning, N. J. & Carter-Su, C. Recent advances in growth hormone signaling. Rev. Endocr. Metab. Disord. 7, 225–235 (2006).
Herrington, J., Smit, L. S., Schwartz, J. & Carter-Su, C. The role of STAT proteins in growth hormone signaling. Oncogene 19, 2585–2597 (2000).
Wang, X., Darus, C. J., Xu, B. C. & Kopchick, J. J. Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation. Mol. Endocrinol. 10, 1249–1260 (1996).
Xu, B. C., Wang, X., Darus, C. J. & Kopchick, J. J. Growth hormone promotes the association of transcription factor STAT5 with the growth hormone receptor. J. Biol. Chem. 271, 19768–19773 (1996).
Hansen, L. H. et al. Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat5 activation. J. Biol. Chem. 271, 12669–12673 (1996).
Ram, P. A., Park, S. H., Choi, H. K. & Waxman, D. J. Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J. Biol. Chem. 271, 5929–5940 (1996).
Smit, L. S. et al. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol. Endocrinol. 10, 519–533 (1996).
Moriggl, R. et al. Deletion of the carboxyl-terminal transactivation domain of MGF-Stat5 results in sustained DNA binding and a dominant negative phenotype. Mol. Cell. Biol. 16, 5691–5700 (1996).
Davey, H. W., Park, S. H., Grattan, D. R., McLachlan, M. J. & Waxman, D. J. STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J. Biol. Chem. 274, 35331–35336 (1999).
Davey, H. W. et al. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142, 3836–3841 (2001).
Kofoed, E. M. et al. Growth hormone insensitivity associated with a STAT5b mutation. N. Engl. J. Med. 349, 1139–1147 (2003).
Scalco, R. C. et al. Growth hormone insensitivity with immune dysfunction caused by a STAT5B mutation in the south of Brazil: evidence for a founder effect. Genet. Mol. Biol. 40, 436–441 (2017).
Zhu, T., Ling, L. & Lobie, P. E. Identification of a JAK2-independent pathway regulating growth hormone (GH)-stimulated p44/42 mitogen-activated protein kinase activity. GH activation of Ral and phospholipase D is Src-dependent. J. Biol. Chem. 277, 45592–45603 (2002).
Ueki, K. et al. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol. Cell. Biol. 22, 965–977 (2002).
Jorgensen, J. O. et al. GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am. J. Physiol. Endocrinol. Metab. 291, E899–E905 (2006).
Krusenstjerna-Hafstrom, T. et al. Insulin and GH signaling in human skeletal muscle in vivo following exogenous GH exposure: impact of an oral glucose load. PLOS ONE 6, e19392 (2011).
Green, H., Morikawa, M. & Nixon, T. A dual effector theory of growth-hormone action. Differentiation 29, 195–198 (1985).
Doi, T. et al. Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I. I. Relationship between increased glomerular size and mesangial sclerosis. Am. J. Pathol. 137, 541–552 (1990).
Lupu, F., Terwilliger, J. D., Lee, K., Segre, G. V. & Efstratiadis, A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 229, 141–162 (2001). These studies systematically address the specific contribution of IGF1 versus GH on linear growth.
Dietz, J. & Schwartz, J. Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism 40, 800–806 (1991).
Richelsen, B. et al. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism 49, 906–911 (2000).
Doris, R., Vernon, R. G., Houslay, M. D. & Kilgour, E. Growth hormone decreases the response to anti-lipolytic agonists and decreases the levels of Gi2 in rat adipocytes. Biochem. J. 297, 41–45 (1994).
Yip, R. G. & Goodman, H. M. Growth hormone and dexamethasone stimulate lipolysis and activate adenylyl cyclase in rat adipocytes by selectively shifting Gi alpha2 to lower density membrane fractions. Endocrinology 140, 1219–1227 (1999).
Ottosson, M. et al. Growth hormone inhibits lipoprotein lipase activity in human adipose tissue. J. Clin. Endocrinol. Metab. 80, 936–941 (1995).
Zhao, J. T. et al. Identification of novel GH-regulated pathway of lipid metabolism in adipose tissue: a gene expression study in hypopituitary men. J. Clin. Endocrinol. Metab. 96, E1188–E1196 (2011).
Puri, V. et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl Acad. Sci. USA 105, 7833–7838 (2008).
Nielsen, T. S. et al. Fasting, but not exercise, increases adipose triglyceride lipase (ATGL) protein and reduces G(0)/G(1) switch gene 2 (G0S2) protein and mRNA content in human adipose tissue. J. Clin. Endocrinol. Metab. 96, E1293–E1297 (2011).
Pedersen, M. H. et al. Substrate metabolism and insulin sensitivity during fasting in obese human subjects: impact of GH blockade. J. Clin. Endocrinol. Metab. 102, 1340–1349 (2017).
Kaltenecker, D. et al. Adipocyte STAT5 deficiency promotes adiposity and impairs lipid mobilisation in mice. Diabetologia 60, 296–305 (2017).
Nordstrom, S. M., Tran, J. L., Sos, B. C., Wagner, K. U. & Weiss, E. J. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol. Endocrinol. 27, 1333–1342 (2013).
Shi, S. Y. et al. Adipocyte-specific deficiency of Janus kinase (JAK) 2 in mice impairs lipolysis and increases body weight, and leads to insulin resistance with ageing. Diabetologia 57, 1016–1026 (2014).
Slayton, M., Gupta, A., Balakrishnan, B. & Puri, V. CIDE proteins in human health and disease. Cells 8, 238 (2019).
Rubio-Cabezas, O. et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol. Med. 1, 280–287 (2009).
Tanaka, N. et al. Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. J. Biol. Chem. 290, 3092–3105 (2015).
Zandbergen, F. et al. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem. J. 392, 313–324 (2005).
Burgermeister, E. et al. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor γ. Mol. Cell. Biol. 27, 803–817 (2007).
Banks, A. S. et al. An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature 517, 391–395 (2015).
Choi, J. H. et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466, 451–456 (2010).
Li, P. et al. Adipocyte NCoR knockout decreases PPARγ phosphorylation and enhances PPARγ activity and insulin sensitivity. Cell 147, 815–826 (2011).
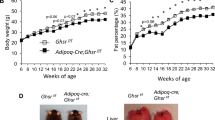
List, E. O. et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol. Endocrinol. 27, 524–535 (2013).
List, E. O. et al. Adipocyte-specific GH receptor-null (AdGHRKO) mice have enhanced insulin sensitivity with reduced liver triglycerides. Endocrinology 160, 68–80 (2019).
List, E. O. et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 155, 1793–1805 (2014).
Berryman, D. E. et al. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm. IGF. Res. 14, 309–318 (2004).
Palmer, A. J. et al. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology 150, 1353–1360 (2009).
Bartke, A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell 7, 285–290 (2008).
Berryman, D. E. et al. Two-year body composition analyses of long-lived GHR null mice. J. Gerontol. A. Biol. Sci. Med. Sci. 65, 31–40 (2010).
Heiman, M. L., Tinsley, F. C., Mattison, J. A., Hauck, S. & Bartke, A. Body composition of prolactin-, growth hormone, and thyrotropin-deficient Ames dwarf mice. Endocrine 20, 149–154 (2003).
Junnila, R. K. et al. Disruption of the GH receptor gene in adult mice increases maximal lifespan in females. Endocrinology 157, 4502–4513 (2016).
Luque, R. M. et al. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLOS ONE 6, e15767 (2011).
Hill, C. M. et al. Long-lived hypopituitary Ames dwarf mice are resistant to the detrimental effects of high-fat diet on metabolic function and energy expenditure. Aging Cell 15, 509–521 (2016).
Berryman, D. E., Lubbers, E. R., Magon, V., List, E. O. & Kopchick, J. J. A dwarf mouse model with decreased GH/IGF-1 activity that does not experience life-span extension: potential impact of increased adiposity, leptin, and insulin with advancing age. J. Gerontol. A Biol. Sci. Med. Sci. 69, 131–141 (2014).
Berryman, D. E. et al. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology 147, 2801–2808 (2006).
Olsson, B. et al. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology 146, 920–930 (2005).
Robertson, K., Kopchick, J. J. & Liu, J. L. Growth hormone receptor gene deficiency causes delayed insulin responsiveness in skeletal muscles without affecting compensatory islet cell overgrowth in obese mice. Am. J. Physiol. Endocrinol. Metab. 291, E491–E498 (2006).
Yang, T. et al. Growth hormone receptor antagonist transgenic mice are protected from hyperinsulinemia and glucose intolerance despite obesity when placed on a HF diet. Endocrinology 156, 555–564 (2015).
Silha, J. V. et al. Perturbations in adiponectin, leptin and resistin levels in acromegaly: lack of correlation with insulin resistance. Clin. Endocrinol. 58, 736–742 (2003).
Ueland, T. et al. Associations between body composition, circulating interleukin-1 receptor antagonist, osteocalcin, and insulin metabolism in active acromegaly. J. Clin. Endocrinol. Metab. 95, 361–368 (2010).
Fain, J. N., Ihle, J. H. & Bahouth, S. W. Stimulation of lipolysis but not of leptin release by growth hormone is abolished in adipose tissue from Stat5a and b knockout mice. Biochem. Biophys. Res. Commun. 263, 201–205 (1999).
Kanety, H. et al. Total and high molecular weight adiponectin are elevated in patients with Laron syndrome despite marked obesity. Eur. J. Endocrinol. 161, 837–844 (2009).
Nilsson, L. et al. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem. Biophys. Res. Commun. 331, 1120–1126 (2005).
White, U. A., Maier, J., Zhao, P., Richard, A. J. & Stephens, J. M. The modulation of adiponectin by STAT5-activating hormones. Am. J. Physiol. Endocrinol. Metab. 310, E129–E136 (2016).
Eden Engstrom, B., Burman, P., Holdstock, C. & Karlsson, F. A. Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH-deficient patients. J. Clin. Endocrinol. Metab. 88, 5193–5198 (2003).
Reyes-Vidal, C. et al. Prospective study of surgical treatment of acromegaly: effects on ghrelin, weight, adiposity, and markers of CV risk. J. Clin. Endocrinol. Metab. 99, 4124–4132 (2014).
Ray, H., Pinteur, C., Frering, V., Beylot, M. & Large, V. Depot-specific differences in perilipin and hormone-sensitive lipase expression in lean and obese. Lipids Health Dis. 8, 58 (2009).
Freda, P. U. et al. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J. Clin. Endocrinol. Metab. 93, 2334–2343 (2008).
Bengtsson, B. A. et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J. Clin. Endocrinol. Metab. 76, 309–317 (1993).
Johannsson, G. et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J. Clin. Endocrinol. Metab. 82, 727–734 (1997).
Benencia, F. et al. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology 156, 1794–1803 (2015).
Stout, M. B. et al. Transcriptome profiling reveals divergent expression shifts in brown and white adipose tissue from long-lived GHRKO mice. Oncotarget 6, 26702–26715 (2015).
Berryman, D. E. & List, E. O. Growth hormone’s effect on adipose tissue: quality versus quantity. Int. J. Mol. Sci. 18, 1621 (2017).
Flint, D. J., Binart, N., Kopchick, J. & Kelly, P. Effects of growth hormone and prolactin on adipose tissue development and function. Pituitary 6, 97–102 (2003).
Hjortebjerg, R. et al. Insulin, IGF-1, and GH receptors are altered in an adipose tissue depot-specific manner in male mice with modified GH action. Endocrinology 158, 1406–1418 (2017).
Gude, M. F. et al. PAPP-A, IGFBP-4 and IGF-II are secreted by human adipose tissue cultures in a depot-specific manner. Eur. J. Endocrinol. 175, 509–519 (2016).
Hjortebjerg, R. et al. Depot-specific and GH-dependent regulation of IGF binding protein-4, pregnancy-associated plasma protein-A, and stanniocalcin-2 in murine adipose tissue. Growth Horm. IGF Res. 39, 54–61 (2018).
Boucher, J. et al. Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes 65, 2201–2213 (2016).
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. EBioMedicine 21, 21–28 (2017).
Kuilman, T. & Peeper, D. S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 9, 81–94 (2009).
McHugh, D. & Gil, J. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 217, 65–77 (2018).
Xu, L. et al. Improves the in vitro developmental competence and reprogramming efficiency of cloned bovine embryos by additional complimentary cytoplasm. Cell. Reprogram. 21, 51–60 (2019).
Villaret, A. et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 59, 2755–2763 (2010).
Crewe, C., An, Y. A. & Scherer, P. E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82 (2017).
Stout, M. B., Justice, J. N., Nicklas, B. J. & Kirkland, J. L. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology 32, 9–19 (2017).
Stout, M. B. et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging 6, 575–586 (2014).
Comisford, R. et al. Growth hormone receptor antagonist transgenic mice have increased subcutaneous adipose tissue mass, altered glucose homeostasis and no change in white adipose tissue cellular senescence. Gerontology 62, 163–172 (2016).
Bogazzi, F. et al. Growth hormone is necessary for the p53-mediated, obesity-induced insulin resistance in male C57BL/6J x CBA mice. Endocrinology 154, 4226–4236 (2013).
Tchkonia, T. et al. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684 (2010).
Palmer, A. K. et al. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64, 2289–2298 (2015).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
Pasarica, M. et al. Adipose tissue collagen VI in obesity. J. Clin. Endocrinol. Metab. 94, 5155–5162 (2009).
Householder, L. A. et al. Increased fibrosis: a novel means by which GH influences white adipose tissue function. Growth Horm. IGF Res. 39, 45–53 (2018).
Hagberg, C. E. et al. Flow cytometry of mouse and human adipocytes for the analysis of browning and cellular heterogeneity. Cell Rep. 24, 2746–2756.e5 (2018).
Gao, H. et al. CD36 is a marker of human adipocyte progenitors with pronounced adipogenic and triglyceride accumulation potential. Stem Cells 35, 1799–1814 (2017).
Varlamov, O., Chu, M., Cornea, A., Sampath, H. & Roberts, C. T. Jr. Cell-autonomous heterogeneity of nutrient uptake in white adipose tissue of rhesus macaques. Endocrinology 156, 80–89 (2015).
Seydoux, J. et al. Adrenoceptor heterogeneity in human white adipocytes differentiated in culture as assessed by cytosolic free calcium measurements. Cell. Signal. 8, 117–122 (1996).
Gliemann, J. & Vinten, J. Lipogenesis and insulin sensitivity of single fat cells. J. Physiol. 236, 499–516 (1974).
Salans, L. B. & Dougherty, J. W. The effect of insulin upon glucose metabolism by adipose cells of different size. Influence of cell lipid and protein content, age, and nutritional state. J. Clin. Invest. 50, 1399–1410 (1971).
Sanchez-Gurmaches, J. & Guertin, D. A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5, 4099 (2014).
Chau, Y. Y. et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 16, 367–375 (2014).
Bluher, M., Patti, M. E., Gesta, S., Kahn, B. B. & Kahn, C. R. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J. Biol. Chem. 279, 31891–31901 (2004).
Xia, B. et al. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLOS Genet. 13, e1007110 (2017).
Lee, K. Y. et al. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 38, e99291 (2019).
Min, S. Y. et al. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc. Natl Acad. Sci. USA 116, 17970–1797 (2019).
Acknowledgements
J.J.K. acknowledges the support of the state of Ohio’s Eminent Scholar Program, which includes a gift from Milton and Lawrence Goll and AMVETS. J.J.K. and D.E.B. acknowledge the support of NIH/NIA AG059779, The Edison Biotechnology Institute and Diabetes Institute at Ohio University. V.P. acknowledges the support of NIH/NIDDK grant DK10171, NIH/NHLBI HL139049 and funds from Osteopathic Heritage Foundation’s Vision 2020 to Heritage College of Osteopathic Medicine at Ohio University. K.Y.L. acknowledges the support of start-up funds from Ohio University Heritage College of Osteopathic Medicine, the Ohio University Diabetes Institute and the American Diabetes Association Junior Faculty Development Award 1–17-JDF-055.
Author information
Authors and Affiliations
Contributions
J.J.K., V.P., D.E.B., K.Y.L. and J.O.L.J. wrote the manuscript. J.J.K., V.P., D.E.B., K.Y.L. and J.O.L.J. surveyed the literature, substantially contributed to related discussions and carried out review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks S. Melmed and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- GH deficiency
-
(GHD). A rare disorder characterized by the inadequate secretion of growth hormone (GH); GHD can be categorized into congenital or acquired, and/or childhood or adult onset.
- Panhypopituitarism
-
Also known as hypopituitarism. Inadequate production or absence of anterior pituitary hormones.
- Acromegaly
-
A condition caused by hypersecretion of growth hormone from a pituitary tumour that is managed by surgical tumour removal or medical control.
- Glomerulosclerosis
-
A degenerative process that involves scarring within the renal glomeruli of the kidney.
- Laron syndrome
-
A rare condition of growth hormone resistance characterized by short stature, which is often caused by mutations in the GHR gene and is inherited in an autosomal recessive manner.
- Senolytic agents
-
Small molecules that can selectively target and kill senescent cells.
Rights and permissions
About this article
Cite this article
Kopchick, J.J., Berryman, D.E., Puri, V. et al. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol 16, 135–146 (2020). https://doi.org/10.1038/s41574-019-0280-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0280-9
This article is cited by
-
An organism-wide atlas of hormonal signaling based on the mouse lemur single-cell transcriptome
Nature Communications (2024)
-
Polycystic ovary syndrome preceding the diagnosis of acromegaly: a retrospective study in 97 reproductive-aged women
Reproductive Biology and Endocrinology (2023)
-
New findings on brain actions of growth hormone and potential clinical implications
Reviews in Endocrine and Metabolic Disorders (2023)
-
IGF-1 is positively associated with BMI in patients with acromegaly
Pituitary (2023)
-
Covert actions of growth hormone: fibrosis, cardiovascular diseases and cancer
Nature Reviews Endocrinology (2022)