Abstract
X-linked hypophosphataemia (XLH) is the most common cause of inherited phosphate wasting and is associated with severe complications such as rickets, lower limb deformities, pain, poor mineralization of the teeth and disproportionate short stature in children as well as hyperparathyroidism, osteomalacia, enthesopathies, osteoarthritis and pseudofractures in adults. The characteristics and severity of XLH vary between patients. Because of its rarity, the diagnosis and specific treatment of XLH are frequently delayed, which has a detrimental effect on patient outcomes. In this Evidence-Based Guideline, we recommend that the diagnosis of XLH is based on signs of rickets and/or osteomalacia in association with hypophosphataemia and renal phosphate wasting in the absence of vitamin D or calcium deficiency. Whenever possible, the diagnosis should be confirmed by molecular genetic analysis or measurement of levels of fibroblast growth factor 23 (FGF23) before treatment. Owing to the multisystemic nature of the disease, patients should be seen regularly by multidisciplinary teams organized by a metabolic bone disease expert. In this article, we summarize the current evidence and provide recommendations on features of the disease, including new treatment modalities, to improve knowledge and provide guidance for diagnosis and multidisciplinary care.
Similar content being viewed by others
Introduction
X-linked hypophosphataemia (XLH) is an X-linked dominant disorder caused by mutations in PHEX (located at Xp22.1), which encodes a cell-surface-bound protein-cleavage enzyme (phosphate-regulating neutral endopeptidase PHEX), predominantly expressed in osteoblasts, osteocytes and teeth (odontoblasts and cementoblasts). XLH is the most common cause of inherited phosphate wasting, with an incidence of 3.9 per 100,000 live births and a prevalence ranging from 1.7 per 100,000 children to 4.8 per 100,000 persons (children and adults)1,2,3. Although the pathogenesis of XLH is not fully understood, animal studies indicate that loss of Phex function results in enhanced secretion of the phosphaturic hormone fibroblast growth factor 23 (FGF23), with osteocytes being the primary source of FGF23 production4. These effects explain most of the characteristic features of the disease, including renal phosphate wasting with consequent hypophosphataemia, diminished synthesis of active vitamin D (1,25(OH)2 vitamin D), rickets, osteomalacia, odontomalacia and disproportionate short stature4,5,6. Patients usually develop clinical symptoms during the first or second year of life. Early treatment with oral phosphate supplementation and active vitamin D heals rickets, limits dental abscess formation and prevents progressive growth failure, but in a substantial proportion of patients treatment is unsuccessful and/or associated with adverse effects (for example, hyperparathyroidism and nephrocalcinosis)7,8. Up to two-thirds of children with XLH require lower limb surgery9,10,11,12. Conventional therapy further stimulates FGF23 levels and thereby renal phosphate wasting, resulting in a vicious circle, which might limit its efficacy6,13,14,15. Adult patients with XLH are at risk of complications such as early osteoarthritis, enthesopathies, spinal stenosis, pseudofractures and hearing loss, which might limit quality of life16,17,18. In 2018, burosumab, a fully human monoclonal IgG1 antibody neutralizing FGF23, was approved by health authorities for the treatment of patients with XLH in the European Union and the USA on the basis of encouraging clinical trial results19,20,21,22,23,24,25. Treatment with phosphate and/or active vitamin D does not decrease or prevent the development of osteoarthritis or enthesopathies17,26. Currently, evidence that treatment with burosumab ameliorates these complications is lacking.
Owing to the rarity of XLH and the diversity of clinical manifestations, diagnosis is often delayed and treatment can be challenging. To date, except for brief guidance provided by two expert groups, no evidence-based, systematically developed recommendations for the diagnosis and management of XLH exist7,8. Therefore, an initiative to develop recommendations for the diagnosis and management of patients with XLH was conducted from June 2017 to December 2018, and the recommendations are provided in this Evidence-Based Guideline. These clinical practice recommendations are endorsed by the European Society for Paediatric Nephrology (ESPN), the European Society for Paediatric Endocrinology (ESPE), the European Society of Endocrinology (ESE), the European Reference Network on Rare Endocrine Conditions (Endo-ERN), the European Reference Network on Rare Bone Disorders (BOND), the International Osteoporosis Foundation (IOF) Skeletal Rare Diseases Working Group, the European Calcified Tissue Society (ECTS), the European Paediatric Orthopaedic Society (EPOS) study group on Metabolic and Genetic Bone Disorders, the European Society of Craniofacial Surgery, the European Society for Paediatric Neurosurgery and the European Federation of Periodontology (EFP) and will be revised and endorsed periodically.
Material and methods
Overview of the guideline project
We followed the RIGHT (Reporting Items for Practice Guidelines in Healthcare) Statement for Practice Guidelines27. Two groups were assembled: a core leadership group and a voting panel. The core group comprised specialists from (paediatric) endocrinology (D.S., M.L.B., L.S., P.K., L.R. and A.L.), paediatric nephrology (D.H., F.E., J.B., D.B., F.S. and E.L.), paediatric orthopaedic surgery (D.E. and P.W.), rheumatology (K.B.), dentistry (M.B.D. and C.C.), neurosurgery (F.D.R.) and XLH patient organization representatives (P.H. and M.K.). The voting group included 41 members with expertise in paediatric and adult XLH, including members of the supporting societies and networks. Voting group members were asked by use of an e-questionnaire to provide a level of agreement on a five-point scale (strongly disagree, disagree, neither agree/disagree, agree or strongly agree) (Delphi method). Failing a 70% level of consensus, recommendations were modified after discussion in the core group and reviewed again by the voting panel until a consensus level of at least 70% was achieved.
Developing the PICO questions
We developed PICO (patient (or population) covered, intervention, comparator and outcomes) questions28. These PICO elements were arranged into the questions to be addressed in the literature searches. Each PICO question then formed the basis for a recommendation.
The population covered included children and adults with XLH. Recommendations have been developed on the basis of available studies investigating the clinical phenotype and management of XLH. For these recommendations, treatment benefits were evaluated using the no treatment option or patient status at baseline (that is, before therapy) as the comparator. With regard to outcomes, we provide recommendations for diagnosis, follow-up and treatment with respect to bone disease, growth, treatment-associated or disease-associated complications and comorbidities.
Literature search
The PubMed database was searched until 26 June 2018; all articles and reports were considered, including prospective randomized controlled trials, uncontrolled or observational studies, registries, summaries and case reports, restricted to human studies in English. The following key MeSH terms were used to identify suitable studies: “X-linked hypophosphatemia”, “X-linked hypophosphatemic rickets”, “hypophosphatemic rickets”, “familial hypophosphatemic rickets”, “PHEX” and “osteomalacia”. The search retrieved 8,903 results and 196 articles were referenced here.
Grading system
We followed the grading system from the American Academy of Pediatrics to develop the recommendations (Fig. 1; refs29,30). The quality of evidence is graded high (A), moderate (B), low (C), very low (D) or not applicable (X). The latter refers to exceptional situations in which validating studies cannot be performed and benefit or harm clearly predominates. This letter was used to grade contraindications for burosumab and safety recommendations for cinacalcet. The strength of a recommendation is graded strong, moderate, weak or discretionary (when no recommendation can be made).
Reproduced with permission from ref.30: Pediatrics 140, e20171904 Copyright © 2017 by the AAP.
Diagnosis
General approach
The diagnosis of XLH is based on the association of clinical, radiological and biochemical findings (Box 1). In patients with a negative family history (approximately one-third of reported patients31,32), mutational analysis of the PHEX gene is recommended, which can provide negative or positive confirmation in ~70–90% of cases31,33,34,35,36,37,38,39,40,41,42,43. Specific molecular genetic defects such as large deletions, deletions removing pseudo-exons of PHEX or mosaicism can be difficult to identify44,45. With atypical clinical presentations and/or negative genetic analyses, further biochemical, molecular genetic and radiological work-up is recommended. The identification of a pathogenic PHEX variant in an index case requires genetic counselling and enables screening of relatives at risk.
Clinical features
In children, the main clinical symptoms of XLH are abnormal gait, lower limb deformity and decreased growth velocity. Dental abscesses are highly prevalent in patients >3 years of age46,47. In undiagnosed adults, typical findings of XLH include short stature, osteomalacia, bone pain, osteoarthritis, pseudofractures, stiffness, enthesopathies and poor dental condition including periodontitis (inflammation of the gums)16,17,18,48,49,50. Osteomalacia-related bone pain needs to be distinguished from osteoarthritis-related bone pain. Rachitic skeletal deformities usually become apparent at the age of 6 months. During the second year of life, patients present with delayed walking, a waddling gait, progressive lower limb deformities (varus deformity or valgus deformity) often combined with a torsional component (intoeing or extoeing), widening of the distal metaphyses at the wrist and ankle level, thickening of the costochondral junctions and reduced growth velocity51. Impaired limb growth with relatively preserved trunk growth results in disproportionate short stature7,8,17,49,52. Patients might present with an abnormal skull shape — that is, dolichocephaly, characterized by parietal flattening, frontal bossing and widened sutures as the consequence of premature fusion of the parietal and frontal bones53,54.
Radiographic manifestations
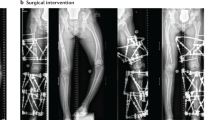
Rachitic lesions are characterized by cupped and flared metaphyses and widened and irregular physes (growth plates) of the long bones. In contrast to rickets that is secondary to vitamin D or calcium deficiency, in XLH, cortical bone often appears thickened and features of bone resorption are lacking. These abnormalities preferentially occur at sites of rapid growth (in particular the distal femora, distal tibiae and distal radii) and typically affect costochondral junctions, which leads to the development of the rachitic rosary and Harrison’s groove. Radiography limited to the knees and/or wrists and/or the ankles is usually sufficient to diagnose rickets8. Bone deformities primarily involve lower limbs (Fig. 2). Adults might present with different radiographic features from children, including pseudofractures, early osteoarthritis of the spine, hip and knees (osteophytes on joint margins or narrowing of joint cartilage) and/or enthesopathies (such as bone proliferation at the site of ligament attachments or calcification of ligaments)16,17. Osteomalacia-related fractures are rarely observed in adults, whereas pseudofractures are frequently observed in adults16,17,55,56.
The patients show disproportionate short stature with genu varum (bowed legs). The final panel on the right shows a patient with a windswept deformity (characterized by a valgus deformity in one knee in association with a varus deformity in the other knee). The radiographs reveal severe leg bowing, partial fraying and irregularity of the distal femoral and proximal tibial growth plates. Note the lack of bone resorption features.
Biochemical characteristics
The biochemical hallmarks of XLH are hypophosphataemia due to renal phosphate wasting, increased alkaline phosphatase (ALP) levels and elevated intact FGF23 levels7,8. A positive family history of XLH, elevated ALP levels, decreased serum phosphate concentrations associated with renal phosphate wasting and/or the identification of a PHEX mutation can help to identify affected children within the first weeks of life. Of note, serum levels of phosphate might be in the normal range within the first 3–4 months of life57,58. Renal phosphate wasting should be evaluated by calculating the tubular maximum reabsorption of phosphate per glomerular filtration rate (TmP/GFR)59 (Table 1). In patients with insufficient phosphate intake, or impaired intestinal absorption (which might be suspected by low urinary levels of phosphate), TmP/GFR can be falsely low until serum levels of phosphate are restored to normal. Although, plasma levels of intact FGF23 are usually elevated, normal levels of FGF23 do not exclude XLH but should be interpreted as inappropriately normal in the setting of hypophosphataemia60. FGF23 levels are also influenced by other factors, in particular phosphate intake and vitamin D therapy2,14,15,60. Therefore, FGF23 levels are most informative in untreated patients6. FGF23 levels are elevated in several other forms of hypophosphataemic rickets (Table 2), and the normal range varies considerably according to the assay used14,61. Serum concentrations of calcium are usually in the lower normal range, and urinary calcium is low owing to impaired 1,25(OH)2 vitamin D synthesis and consequently decreased intestinal calcium absorption. In contrast to calcipenic rickets, parathyroid hormone (PTH) levels are usually at the upper limit of the normal range or even slightly elevated. Circulating levels of 1,25(OH)2 vitamin D are low or inappropriately normal in the setting of hypophosphataemia6,62,63,64.
Genotype–phenotype correlation
Although XLH seems to be completely penetrant, its severity varies widely, even among family members, with no clear gender difference55,65. A large number of inactivating mutations in PHEX can cause XLH, and a genotype–phenotype correlation is not obvious31.
Further patient work-up
Patients should be evaluated for the presence and severity of common and rare complications of XLH depending on their age (Table 1). In young children, dynamic tests or specific investigations, such as hearing evaluation or oral examination, are sometimes not feasible and can be delayed until the child reaches 3–5 years of age.
Other causes of hypophosphataemic rickets
XLH represents ~80% of all cases of hypophosphataemic rickets39,43,45. Other types of hypophosphataemic rickets have similar but not identical clinical and radiological features58. Other causes of hypophosphataemia must be considered in patients with a negative family history, male-to-male transmission or unusual clinical presentation, such as symptoms developing after the second year of life (autosomal dominant hypophosphataemic rickets or tumour-induced osteomalacia), acidosis, glucosuria, aminoaciduria or low molecular mass proteinuria (renal Fanconi syndrome), low urinary phosphate levels (dietary phosphate deficiency or impaired bioavailability) or hypercalciuria before starting treatment (hereditary hypophosphataemic rickets with hypercalciuria (HHRH)) (Table 2). The differential diagnoses are based on the mechanisms leading to hypophosphataemia — namely, high PTH activity, inadequate phosphate absorption from the gut or renal phosphate wasting. The latter might be the result of either primary tubular defects or high levels of circulating FGF23 (refs66,67,68) (Fig. 3; Table 2). Extended molecular genetic analysis can be helpful to establish the diagnosis in unclear cases of hypophosphataemic rickets69,70,71. Nutritional rickets and XLH might sometimes coexist, and diagnosis of XLH should be considered in someone otherwise thought to be vitamin D or calcium deficient if serum levels of phosphate do not improve after supplementation.
The differential diagnoses are based on the mechanisms leading to hypophosphataemia — namely, high parathyroid hormone (PTH) activity, inadequate phosphate absorption from the gut or renal phosphate wasting. The latter may be due to either primary tubular defects or high levels of circulating fibroblast growth factor 23 (FGF23). Further details of individual entities can be found in Table 2. XLH, X-linked hypophosphataemia. Adapted with permission from ref.57, Springer Nature Limited (this material is excluded from the CC-BY-4.0 license).
Follow-up of patients with XLH
We suggest that patients should be seen at regular intervals by multidisciplinary teams organized by an expert in metabolic bone diseases (Box 2). The clinical, biochemical and radiological features and complications of XLH vary widely from patient to patient; therefore, treatment and monitoring should ultimately be tailored to the patient on the basis of their clinical manifestations, medical history, stage of development and the clinician’s professional judgement. The expert should liaise with the patient’s local health-care providers (general practitioners and/or paediatricians), radiologists, orthopaedic surgeons, physical therapists, rheumatologists and dentists. In addition, the following professions might be involved on the basis of individual patient needs: neurosurgeons, otolaryngologists (ENTs), ophthalmologists, orthodontists, dieticians, chiropodists, social workers and psychologists (Table 3).
Clinical evaluation
In children, signs or severity of rickets should be assessed at each visit, including measurement of intercondylar and/or intermalleolar distance, in addition to height and growth velocity72,73. With appropriate conventional therapy (that is, phosphate supplementation and treatment with active vitamin D), rickets should improve, leading to a reduction in limb deformity8, and height should increase by ~1 s.d. within 2–3 years when initiated in preschool-age children8,52,74,75,76. Often, lower limb deformity and joint alignment cannot be assessed fully with clinical measurements alone and radiographic assessment is helpful. Patients with substantial limb deformities should be evaluated by an orthopaedic surgeon with experience in metabolic bone disease. Such an evaluation should include an assessment of limb length and alignment (in both the coronal and sagittal planes) as well as the torsional profile of the lower limb.
Yearly assessment of the 6-minute walk test (6MWT) in patients >5–6 years might help to quantify the functional consequences of XLH on bone and muscles77. Patients should have at least twice-yearly dental examinations after tooth eruption, orthodontic evaluation around the age of 12 years and an extended dental evaluation with transition to adult care. The number of dental abscesses and episodes of acute oral infections (including maxillofacial cellulitis) should be recorded at each visit (as these are indirect indices of impaired tooth mineralization). Craniosynostosis should be considered in children up to the age of 5 years who have an insufficient increase in head circumference, abnormal head shape or neurological signs (including headache and vomiting as a result of increased intracranial pressure)54,78,79. The spine should be examined clinically for lordosis, kyphosis and/or scoliosis. In addition, bone and joint pain, disability and fatigue should be assessed by patient-reported outcomes.
Biochemical follow-up
Serum level of ALP is a reliable biomarker of rickets activity and osteomalacia in children and adults8,80,81,82,83. Given that bone-specific ALP represents ~80–90% of total ALP in the serum of children, total ALP might be used in this population. In adults, bone-specific ALP is preferred given that ~50% of circulating ALP originates from hepatocytes84. When rachitic or osteomalacic bones are undertreated, ALP levels are elevated and urinary levels of calcium are usually low. By contrast, when rickets is healed, ALP levels tend to normalize and urinary calcium levels start to increase7,8. PTH should be measured regularly as secondary hyperparathyroidism is promoted by oral phosphate supplementation64,85,86,87,88. Suppressed PTH levels suggest that the dose of active vitamin D is disproportionately high compared with phosphate supplementation. Measurement of serum and urinary levels of calcium is required to evaluate the safety of active vitamin D. Spot urine samples are preferred as they are simple to perform, especially in young children. Alternatively, 24-hour urine collections can be performed in toilet-trained patients. We do not recommend regular measurement of serum levels of FGF23 in treated patients as it does not guide therapy6,14,89,90.
In patients treated with burosumab, fasting serum phosphate level is a biomarker of efficacy and should be monitored to titrate the treatment in children and to exclude hyperphosphataemia. In some patients, burosumab might initially normalize TmP/GFR while serum level of phosphate is still below the normal range owing to the high demand for phosphate of the bone. In this setting, an increase in burosumab dose will not necessarily result in improved bone healing. Therefore, we suggest that TmP/GFR should be analysed together with fasting serum phosphate levels as a measure of drug efficacy. Serum levels of 1,25(OH)2 vitamin D might increase under burosumab therapy; we suggest measuring these levels every 6 months and analysing them together with the urinary calcium excretion as safety parameters20,91,92,93.
Radiographic and imaging follow-up
Radiographic evaluation is recommended in cases of persistent marked clinical or biochemical signs, for example, elevated levels of ALP despite adequate therapy. Enthesopathies, osteoarthritis and pseudofractures can be accurately diagnosed on plain radiographs and bone nuclear scans17,26,94. The EOS system (which is associated with 50–80% less irradiation than conventional radiography; EOS Imaging, Paris, France) enables 3D assessment of limb deformity95 and should be used whenever possible95. Renal ultrasonography is the preferred method to screen for nephrocalcinosis86,96,97,98,99.
A dental orthopantomogram (radiograph of the upper and lower jaw and teeth) is suggested at 5 years of age and in adults with recent oral manifestations and should be repeated on the basis of clinical needs.
In symptomatic adults and children (such as those with persistent headache, vomiting or abnormal skull shape), we recommend evaluation by brain and/or spinal MRI to exclude craniosynostosis, Chiari 1 malformation or syringomyelia. To avoid excessive irradiation from radiography or computed tomography (CT), the skull can be imaged through the black bone sequence on MRI, which provides a high image contrast between bone and other tissues100.
The clinical value of peripheral quantitative CT (pQCT) or bone mineral density (BMD) measurements by DXA (dual-energy X-ray absorptiometry) in patients with XLH is limited101,102,103. Both methods are unable to diagnose osteomalacia. Intriguingly, trabecular volumetric BMD was even found to be increased in paediatric patients with XLH101,102,103, which might reflect a compensatory mechanism owing to the soft bone.
Elevated arterial blood pressure, left ventricular hypertrophy and/or pathological electrocardiograms have been reported in some but not all studies evaluating the cardiovascular status of patients with XLH104,105,106,107,108,109,110. Therefore, we suggest at least yearly blood pressure measurements, with a more detailed work-up only in the presence of persistently elevated blood pressure.
Conventional treatment in children
Phosphate
Recommendations for conventional treatment in children with XLH are provided in Box 3. Oral phosphate supplements should always be provided together with active vitamin D, as phosphate alone promotes secondary hyperparathyroidism and thereby renal phosphate wasting32,48,111. Treatment doses vary according to age and severity of phenotype, and no consensus exists on the optimal dose of oral phosphate7,8. However, starting doses of 20–60 mg/kg body weight daily (0.7–2.0 mmol/kg daily) based on elemental phosphorus are recommended on the basis of the severity of the phenotype. Early treatment is associated with superior outcomes18,26,32,49,50,75,112,113,114,115. Healing of rickets, as evidenced by the normalization of ALP levels and radiological signs, is the initial aim in children8,32,116. Treatment also promotes growth, reduces bone pain, progressively corrects leg deformities and improves dental health48,50,52,63,74,75,81,82,83,85,111,117. In infants diagnosed before they develop bone changes, the goal of treatment is to prevent rickets. Serum phosphate levels increase rapidly after oral intake but return to baseline concentrations within 1.5 hours63. Thus, phosphate should be given as frequently as possible, for example, 4–6 times per day in young patients with high ALP levels, to maintain stable blood levels. Less frequent dosing (2–3 times daily) might improve adherence in adolescents. Fasting phosphate levels are not restored by oral phosphate supplements, and normalization of serum levels of phosphate is not a goal of conventional therapy7,8. Phosphate supplements are available as oral solutions, capsules or tablets containing sodium-based and/or potassium-based salts. Dosages should always be based on elemental phosphorus given that the phosphorus content largely differs between the available phosphate salts. Oral solutions containing glucose-based sweeteners should be used with caution given the dental fragility of these patients. Phosphate should not be given together with calcium supplements or foods with high calcium content, such as milk, as precipitation in the intestinal tract reduces absorption.
Active and native vitamin D
Active vitamin D (calcitriol or alfacalcidol) is given in addition to oral phosphate supplements in order to counter calcitriol deficiency, prevent secondary hyperparathyroidism and increase phosphate absorption from the gut. The optimal dose varies from patient to patient. Requirements are generally higher during early childhood and puberty (growth phases)51, and the dose can be adjusted on the basis of serum levels of ALP and PTH and urinary calcium excretion. Large doses of active vitamin D promote growth and bone healing but are associated with an increased risk of hypercalciuria and nephrocalcinosis96,97,98,99,118. Conversely, insufficient doses of active vitamin D are usually associated with low intestinal calcium absorption, low urinary calcium excretion, persistent rickets and high ALP and/or PTH levels. Calcitriol can be given in one or two doses per day, whereas alfacalcidol should be give once per day owing to its longer half-life8. The equivalent dosage of alfacalcidol is 1.5–2.0 times that of calcitriol on the basis of studies in patients with secondary hyperparathyroidism due to chronic kidney disease and observations in patients with XLH8,119. This difference is probably because of the roughly twice higher oral bioavailability of calcitriol than alfacalcidol. A single evening dose might help prevent excessive calcium absorption after food intake and thus hypercalciuria120. Several other vitamin D analogues are also available62,117,121,122. As in healthy children, 25(OH) vitamin D deficiency in children with XLH should be corrected by supplementation with native vitamin D.
Calcium supplements
Nutritional calcium intake should be kept within the normal range for age. Supplements are not recommended given that bone mass and mineral content are usually not decreased and because of the potential risk of hypercalciuria101,103,123,124.
Adverse effects of conventional treatment
Conventional treatment with phosphate supplementation and active vitamin D might increase calciuria and thereby promote nephrocalcinosis, which has been reported in 30–70% of patients with XLH3,48,56,87,96,97,98,99,118. Several reports suggest positive associations between daily oral phosphate doses and the risk of developing nephrocalcinosis, whereas the relationship with active vitamin D therapy and/or with the presence of hypercalciuria has been observed less frequently3,48,96,97,99,118,125. Hydrochlorothiazide decreases calciuria in XLH86, and potassium citrate might help prevent calcium precipitation, especially in patients with low urinary citrate levels; however, alkalinization of urine increases the risk of phosphate precipitation. Therefore, potassium citrate should be used with caution in XLH.
Secondary hyperparathyroidism, which might aggravate phosphaturia and promote bone resorption, results from the long-term stimulation of parathyroid cells by FGF23 and phosphate supplements and from the suppression of 1,25(OH)2 vitamin D levels by FGF23, especially in patients not treated with active vitamin D62,64,86,87,88,104,126,127,128. Conversely, suppressed PTH levels secondary to excessive vitamin D therapy and/or insufficient oral phosphate intake might decrease bone turnover and compromise rickets healing and growth. Thus, therapies should be adjusted to keep PTH levels within the normal range (10–65 pg/ml in children and adults). In patients with XLH, adjuvant therapy with a calcimimetic (for example, cinacalcet) decreases serum levels of PTH and FGF23 and increases TmP/GFR126,129,130. Therefore, if PTH levels do not normalize after optimizing active vitamin D (dose increase) and phosphate treatment (dose reduction), cinacalcet might be considered together with close monitoring130. However, cinacalcet is not licensed for this indication and has been associated with severe adverse effects — namely, hypocalcaemia and increased QT interval131. To date, no evidence suggests that burosumab can revert persistent hyperparathyroidism. Therefore, parathyroidectomy should be considered in patients with tertiary hypercalcaemic hyperparathyroidism.
Burosumab in children with XLH
In February 2018, the European Medicines Agency (EMA) granted a conditional marketing authorization in the European Union for burosumab for the treatment of XLH with radiographic evidence of bone disease in children ≥1 year of age and in adolescents with a growing skeleton23. In April 2018, the US Food and Drug Administration (FDA) granted approval of burosumab to treat adults and children ≥1 year with XLH24. These decisions were based on the results of trials testing burosumab in children with severe XLH and in adults with skeletal pain associated with XLH and/or osteomalacia. Serum levels of phosphate, TmP/GFR, the severity of rachitic lesions in children (based on radiography images) and osteomalacia in adults (based on radiography images and bone histomorphometry) were chosen as primary end points. In children, the dose of burosumab was initially titrated against serum levels of phosphate, targeting empirical levels ranging from 1.1 to 1.6 mmol/l, whereas adult patients received a fixed weight-related dose19,22.
Currently only the data submitted to the regulatory agencies and published in peer review journals are available19,132,133. Burosumab is an expensive drug, and data on cost-effectiveness and long-term outcome are pending134,135. Thus, conclusive recommendations on the use of burosumab are premature. However, given the severity of the disease in some patients and the encouraging results that have prompted the EMA and FDA to grant authorization, preliminary recommendations are provided (Box 4).
Two open-label uncontrolled trials testing burosumab in a total of 65 children aged 1–12 years with severe XLH demonstrated that in the short term (12–16 months), burosumab resulted in the following outcomes19,132,133: a statistically significant increase in TmP/GFR and consequently raised serum phosphate levels into the lower end of the age-related normal range, with increased 1,25(OH)2 vitamin D levels; a significant reduction in the severity of rickets (as measured by the Rickets Severity Score (RSS) and the Radiographic Global Impression of Change (RGI-C)); a significant improvement in physical ability (as measured by walking distance in the 6MWT); and a significant reduction in patient-reported pain and functional disability (as measured by the use of the Pediatric Orthopedic Society of North America Outcomes Data Collection Instrument).
The most common adverse reactions observed with burosumab were injection-site reactions, headache and pain in the extremities. Two weekly doses were superior to four weekly doses with respect to normalization of serum levels of phosphate and radiological improvement of rickets. Conventional treatment should be stopped at least 1 week before the start of burosumab therapy for wash-out and to prove that fasting serum phosphate levels are below the normal reference for age.
The EMA and FDA authorizations approved a starting dose of 0.4 mg/kg body weight and 0.8 mg/kg body weight, respectively, given every 2 weeks132,133. We suggest starting with a dose of 0.4 mg/kg body weight as this dose might be sufficient. The dose should be titrated in increments of 0.4 mg/kg body weight in order to raise fasting serum phosphate levels within the lower end of the normal reference range for age, with a maximum dosage of 2.0 mg/kg body weight (maximum dose 90 mg). In paediatric trials, the average maintenance dose was 1 mg/kg body weight19.
Pharmacokinetic and pharmacodynamic studies showed similar results in children and adults, with a drug half-life of ~19 days and peak serum concentrations of burosumab at 7–11 days after injection, which paralleled with the increase in serum levels of phosphate and TmP/GFR, thus supporting a direct pharmacokinetic–pharmacodynamic relationship133. We therefore suggest monitoring fasting serum phosphate levels during the titration period between injections, ideally 7–11 days after the last injection, to avoid inadvertently causing hyperphosphataemia. After achievement of a steady state, which can be assumed after 3 months of a stable dosage, we suggest monitoring serum levels of phosphate preferentially directly before injections to detect hypophosphataemia. Burosumab should not be adjusted more frequently than every 4 weeks, and longer intervals of at least 2 months are suggested. Burosumab must not be given when phosphate levels are within the age-related normal reference range before initiation of treatment or in the presence of severe renal impairment (as these patients are at risk of developing hyperphosphataemia). Targeting fasting serum phosphate in the lower end of the normal reference range for age is probably the safest approach to limit the risk of ectopic mineralization.
Growth hormone
Final heights are reduced in up to 60% of patients with XLH despite conventional treatment8,48,49,50,52,56,75,76,136,137,138,139. Administration of recombinant human growth hormone (rhGH) resulted in a sustained increase in age-standardized height during treatment periods of up to 3 years, and prepubertal children responded better to rhGH than pubertal patients140. Administration of rhGH was associated with a transient increase in serum levels of phosphate and PTH. A 3-year randomized controlled trial in severely short children with XLH showed substantial growth response in comparison to controls individuals, without aggravation of body disproportion141. However, long-term follow-up of the same study failed to show significant benefits on the adult height, whereas in another study mean final height was significantly higher than in patients who did not receive rhGH142,143 (Box 5).
Conventional treatment in adults
Treatment is recommended in symptomatic adult patients with XLH — that is, those with musculoskeletal pain, pseudofractures, dental issues, planned orthopaedic or dental surgery or biochemical evidence of osteomalacia with an increase in serum levels of bone-specific ALP (Box 6). Conventional treatment with active vitamin D and phosphate improves pain, osteomalacia and oral health (with respect to periodontitis and the frequency of dental abscesses) but does not prevent or improve hearing loss or enthesopathies.
In addition to oral health18, little evidence suggests that starting or continuing treatment in asymptomatic adults improves outcomes8,16,17,56,122,144,145,146. Taking daily active vitamin D and at least twice-daily oral phosphate supplements is burdensome for many adults and has potential adverse effects16,17,18,26,56,113,114,122,144.
Calcitriol or alfacalcidol doses that are usually prescribed in adults range from 0.50 to 0.75 μg daily for calcitriol and 0.75–1.5 μg daily for alfacalcidol. Phosphate supplements, available as oral solutions, capsules or tablets, containing sodium-based or potassium-based salts, should be given at 750–1,600 mg daily (based on elemental phosphorus) in 2–4 divided doses26,82,83,114,115. To avoid gastrointestinal adverse effects, the dose of phosphorus should be increased gradually. Theoretically, potassium-based phosphate salts could decrease the risk of hypercalciuria compared with sodium-based preparations. Taking active vitamin D, which increases calcium absorption, in the evening might reduce intestinal calcium absorption3,16,17,26,82,83,86,87,96,99,114,122,124,144,147. Vitamin D deficiency should be corrected as in the general population. Thiazide diuretics have been suggested to increase renal calcium reabsorption and to enhance bone mineralization; however, the long-term effects of this treatment are not known, as discussed in the treatment for children section98,148. Normal calcium intake (minimum 1g per day) and a low-sodium diet are recommended to reduce calciuria and support weight control.
Pregnancy is a critical moment for bone health. Therefore, during pregnancy, 25(OH) vitamin D levels should be monitored and adjusted. Phosphate supplementation might require higher dosages, up to 2,000 mg daily. Most patients already on therapy will simply continue their treatment. We also suggest considering conventional therapy for women with XLH who are not on therapy at the time of conception. All treated pregnant women should undergo close biochemical monitoring. Women are encouraged to breastfeed if they want to, regardless of treatment. Conventional therapy is suggested in lactating women to prevent bone loss149.
Orthopaedic procedures are usually indicated to correct deformity (both angular and torsional) and for the treatment of pathological fractures. In adults, these conditions are unlikely to improve with medical management alone, which should nonetheless always accompany surgical management16,144,150,151,152. In patients undergoing orthopaedic surgery, therapy might need to be discontinued if long-term bed rest and/or non-weight-bearing mobilization is anticipated to avoid hypercalciuria and/or hypercalcaemia due to increased bone resorption80,99.
Burosumab in adult patients
One open-label, uncontrolled trial and one randomized, double blind, placebo-controlled study (including a total of 148 patients) have investigated burosumab in adults with skeletal pain associated with XLH and/or osteomalacia25,132,133. Short-term treatment (6–12 months) with burosumab was associated with the following outcomes25,132,133: significantly increased TmP/GFR and consequently raised serum levels of phosphate into the lower normal range and increased 1,25(OH)2 vitamin D levels; healed osteomalacia and accelerated healing of active fractures and pseudofractures; and significantly reduced stiffness (as measured by the Western Ontario and the McMaster Universities Osteoarthritis Index (WOMAC) stiffness subscale). By contrast, reductions in WOMAC physical function subscale and the Brief Pain Inventory score did not achieve statistical significance when compared with placebo.
Notably, all studies to date in adults with XLH took place in moderately to severely affected patients, making extrapolation to real-world patients difficult as many real-world patients might have mild disease. The reported adverse events were similar to those observed in the paediatric trials (that is, injection-site reactions, headache and pain in the extremities). In the USA, the FDA approved a dose of burosumab of 1 mg/kg body weight, with a maximum dose of 90 mg, given subcutaneously every 4 weeks132. General monitoring during burosumab treatment is given in Table 3. Owing to the pharmacokinetic and pharmacodynamic characteristics of burosumab, we suggest initially monitoring fasting serum phosphate levels between injections, ideally 7–11 days after the last injection, to avoid inadvertently causing hyperphosphataemia (Box 7). After achievement of a steady state, which can be assumed after 3 months, we suggest measuring serum levels of phosphate, preferentially during the last week before the next injection, to detect underdosing133. Otherwise, the contraindications and modifications in patient monitoring are the same as in children.
Musculoskeletal symptoms
Muscle strength is considerably lower in patients with XLH than in healthy control individuals, without changes in muscle cross-sectional area102,153,154. Enthesopathies are prevalent in adult XLH and are usually detectable by the third decade of life; conventional therapy does not seem to prevent or treat these complications26,155,156. Musculoskeletal symptoms represent a high burden in adults with XLH, including stiffness due to joint involvement and/or the presence of enthesopathies, muscle weakness, fatigue and physical deconditioning and pain. These symptoms result in mobility impairment, reduced physical activity and reduced quality of life, which is a strong rationale for prescribing non-pharmacological treatments and self-management17. The goals of physical therapy are to provide pain relief, to improve physical function and fitness and to reduce XLH-related disability (Box 8). To date, no disease-specific recommendations exist for physical therapy in patients with XLH, and programmes are based on recommendations of physical therapies for individuals with knee or hip osteoarthritis.
Orthopaedic management of XLH in children
Osteotomies performed early in childhood to treat deformity have historically been associated with a high recurrence rate and complications11,157,158,159. Currently, with improvements in medical care, limb deformity improves in most patients, persists in others and progresses to a severe deformity in a minority9,10,52,160. Surgical approaches now include the less invasive techniques of guided growth surgery performed early in childhood, which contrast with the active delay of corrective surgery using complex osteotomies until the patient is skeletally mature9,160. In guided growth surgery, a small metal plate is placed on the medial or lateral surface of the bone (for valgus or varus deformities) at the level of the physis (growth plate). A screw is placed into the bone at either side of the growth plate, and the device then acts as a tether to growth near the plate but not at the opposite side of the bone. Thus, with time, the differential growth allows the alignment to improve, and when the bone is straight, the plate is removed and normal growth continues.
This change in both medical and surgical treatment philosophies makes it difficult to make firm recommendations at this stage161,162,163,164 (Box 9).
Casts, insoles and physiotherapy
Evidence does not support the use of insoles or casts to improve lower limb deformity associated with XLH. Insoles cannot improve the position of a flat foot if this is distal to a valgus knee. Physiotherapy (in terms of a general strengthening and/or a gait education programme) might be helpful, especially after surgery.
Orthopaedic surgery
The radiographic indications for elective surgical treatment include, in the coronal plane, a deviation of the mechanical axis into zones 3 or 4 (refs160,165). In children who are still growing, a mechanical axis that is progressive through zone 2 despite optimized medical care might also merit treatment160. Treatment at this stage is often simpler, more reliable and less prone to complications than treatment when patients have stopped growing. Substantial deformity might also exist in the sagittal and torsional planes. Deformity in three planes increases the difficulty of any surgical intervention, but often the coronal deformity dominates and correction of this plane improves the overall picture160,165,166.
The aim of surgical treatment is that at skeletal maturity the lower limbs are of equal length, well aligned (that is, with neutral lower limb mechanical axes and horizontal knee and ankle joints) and with mobile, comfortable joints. If possible, this goal should be achieved with the minimum amount of surgery, no complications, minimal time off school and/or activities and no functional loss.
Lower limb deformities can be treated by performing osteotomies at the site or sites of major deformity and correcting in all three planes. The osteotomy could result in acute correction of the deformity with internal fixation or gradual correction of deformity using external fixation techniques9,117,122,159,160,167. Such surgery is associated with notable rates of recurrence and of complications, especially in young children and in patients with poor metabolic control. In one study, 57% patients (28 of 49) experienced at least one complication of surgery, with recurrent deformity in 29% of patients9. Therefore, delaying surgical treatment for residual deformity until skeletal maturity might be prudent. Rarely, major deformities can induce severe knee instability, and these patients should undergo osteotomies before they stop growing.
Guided growth techniques have been gaining popularity165. This surgical strategy should commence early after 12 months if deformity persists despite maximized medical therapy. The rationale for this therapy is to correct the deformity at the physis before significant diaphyseal deformity develops. With correction of the mechanical axis, the forces directed across the physes and the joints are normalized, thus promoting normal growth. This technique does not require immobilization. The limitation of this strategy is that it provides only uniplanar coronal correction without correction of the tibial medial torsion. Torsional alignment often improves simultaneously. Both varus and valgus deformities correct readily and rapidly, but genu varum might respond less in adolescents160. In growing children, guided growth techniques might lead to overcorrection if the plates are left in situ for too long. Rebound deformity after plate removal has been reported, although rarely12,160,168,169.
Whether or not deformity correction at the knee results in some correction distally at the ankle is debated160,168. If early treatment could reduce deformity at distal joints, then the overall requirement for surgical procedures might be reduced. Similarly, if the limb alignment overall is satisfactory and the joints are horizontal, it is unclear as to whether residual minor diaphyseal deformity or torsional deformity causes significant functional difficulties.
Assessment of surgical outcomes
Postoperative assessment of the axis correction should be documented at 12 months. Functional assessment should be performed according to the World Health Organization (WHO) International Classification of Function using tools such as the Pediatric Outcomes Data Collection Instrument (PODCI) or the 6MWT77,170. As patients transition to adult services, a full orthopaedic clinical and radiographic assessment enables definition of any residual deformity and facilitates appropriate follow-up arrangements.
Dental health
Endodontic infections
Children and adult patients with XLH might present with spontaneous endodontic infections on apparently intact teeth18,114,115,171. Dental abscesses can develop on deciduous as well as on permanent teeth16,171. The endodontic infection might be asymptomatic for months or years or it might evolve into dental abscesses, causing pain and swelling. Spread to surrounding anatomical structures causes maxillofacial cellulitis.
Dental complications in patients with XLH are secondary to poorly mineralized dentin. On dental radiographs, the pulp chambers of deciduous or permanent teeth are larger than usual with long pulp horns extending to the dentino–enamel junction114,172. XLH is not associated with increased susceptibility to caries114,115,171.
Periodontitis
The frequency and severity of periodontitis is increased in adult patients with XLH and can lead to tooth loss. Cementum thickness, a central component of the periodontium, is reduced and associated with mineralization defects of the alveolar bone, which impairs the attachment of the ligament fibres18,171. On radiographs from adult patients, the lamina dura might be absent and the alveolar bone around teeth is frequently reduced18,173.
Prophylaxis
Conventional treatment improves dentin mineralization, reduces the number of dental abscesses and decreases the frequency and severity of periodontitis18,114 (Box 10). Reducing the size of the pulp chambers, as demonstrated by dental radiographs, is a useful sign of successful treatment. Continued supplementation through adulthood may be beneficial, as the number of dental abscesses and severity of periodontitis vary according to the percentage of adult life with treatment18,26.
To prevent bacterial invasion of the dentin and pulp via enamel microcracks, we suggest sealing the occlusal surfaces of both deciduous and permanent teeth in children with XLH174. This approach can be achieved with flowable resin composite placed in the pits and fissures of the occlusal surface and should be performed regularly as soon as the eruption of the tooth allows acceptable isolation of the occlusal surface.
Acute abscesses might require antibiotic treatment depending on the extent and severity of the infection. For deciduous teeth, the decision to extract or treat endodontically will depend on the extent of the infection, recurrence and the expected timing of normal exfoliation of the permanent tooth. For permanent teeth, endodontic treatment or re-treatment of the tooth are the preferred options, although healing after endodontic treatment might not be as favourable as in healthy patients.
Prevention, treatment and supportive care of periodontitis in adult patients should follow standard management, as in the general population. Treatment of periodontitis should aim to decrease gingival inflammation and suppress periodontal pockets. The opportunity to resume or start conventional medical treatment should be discussed in the presence of periodontitis18.
Orthodontic and implant treatments
Children with XLH often present with delayed dental development, abnormal eruption patterns, increased frequency of specific malocclusions (including maxillary retrognathism) and impacted or dystopic maxillary canines175,176,177. Orthodontic treatment triggers movement of the teeth and extensive remodelling of the alveolar bone. Without conventional treatment, the outcomes of orthodontic treatment are unpredictable. The high rate of tooth loss secondary to endodontic infections and periodontitis often leads to the need for dental implants in adults with XLH178,179. Standard dental surgical protocols in adults with XLH who are not receiving conventional treatment result in decreased success rates compared with healthy control individuals180.
Hearing loss
Impaired hearing has been observed in patients with XLH as early as 11 years of age, including subjective hearing loss, episodic tinnitus, deafness and vertigo181,182. Symptomatic patients present with generalized osteosclerosis and thickening of the petrous bone, and moderate internal auditory meatus narrowing, particularly in its mid-portion, compared with healthy control individuals181,182. Treatment is similar as for other causes of hearing loss and includes hearing aids, prevention of noise exposure and avoidance of ototoxic drugs (Box 11).
Neurosurgical complications
Craniosynostosis
Recommendations for the management of neurosurgical complications can be found in Box 12. Craniosynostosis can occur as early as 1 year of age and usually involves an abnormal fusion of the sagittal suture leading to a dolichocephalic conformation of the head with a reduced cranial index54,79. When systematically investigated with skull radiographic examination, suture fusion is identified in ~60% of children with XLH79,183. Craniosynostosis should be suspected in the presence of signs of intracranial hypertension, such as headache, neck pain or papilledema54,78,79,184,185. However, children might be asymptomatic, despite increased intracranial pressure78,79,185. An abnormal shape of the skull without any clinical symptoms does not necessarily mandate performing an MRI, and decisions should be made on an individual basis.
Chiari type 1 malformation
Chiari type 1 malformation, which causes prolapse of the cerebellar tonsils through the foramen magnum, is detected in 25–50% of children with XLH by use of cranial MRI or CT54,79,183,186. Most cases are asymptomatic, but compression of the lower brainstem and upper cervical cord might cause symptoms and/or result in syringomyelia requiring surgical correction. Symptoms can include occipital or neck pain exacerbated by Valsalva manoeuvres, peripheral motor and/or sensory defects, clumsiness, hyporeflexia or hyperreflexia, respiratory irregularities and central apnoeas and lower cranial nerve dysfunction54,79.
Lifestyle
Evidence suggests that glucose and lipid metabolism might be compromised in individuals with XLH, resulting in obesity127,128,187,188. Adolescent and adult patients with XLH are prone to develop obesity partly because rheumatological and chronic bone complications decrease the propensity of patients to exercise16,17,56,109,113,189 (Box 13).
Potential new treatments
In addition to antibodies directed against FGF23, other drugs aiming at inhibiting the effects of high FGF23 levels are under development, some of which might enter clinical use in future years. For example, the FGF23 receptor antagonist NVP-BGJ398 is in an advanced phase of development190. A number of other potential pharmacological targets have also been identified, including antagonizing peptides known to inhibit bone mineralization and regulation of FGF23 protein expression190,191,192.
Conclusions and perspectives
XLH is a rare chronic disease that substantially alters the quality of life of affected patients throughout life. Knowledge of this condition is unfortunately often restricted to a few specialized centres. This multisystem disease evolves over time, and multidisciplinary care of patients with XLH is needed, involving physicians, physiotherapists, dentists and social workers and liaison with patient group representatives. In these recommendations, we have attempted to cover most features of the disease in order to support such lifelong multidisciplinary care of patients with XLH. Our aim was to identify the criteria for diagnosis, provide guidance for medical and surgical treatment and explain the challenges of follow-up. These recommendations will be updated in the future, in particular when more information on the natural history of the disease becomes available and further data on burosumab emerge. Various topics for future research are outlined in Box 14.
References
Beck-Nielsen, S. S., Brock-Jacobsen, B., Gram, J., Brixen, K. & Jensen, T. K. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur. J. Endocrinol. 160, 491–497 (2009).
Endo, I. et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: prevalence, biochemical data and treatment. Endocr. J. 62, 811–816 (2015).
Rafaelsen, S., Johansson, S., Ræder, H. & Bjerknes, R. Hereditary hypophosphatemia in Norway: a retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur. J. Endocrinol. 174, 125–136 (2016).
Liu, S. et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–E49 (2006).
Feng, J. Q., Clinkenbeard, E. L., Yuan, B., White, K. E. & Drezner, M. K. Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia. Bone 54, 213–221 (2013).
Carpenter, T. O. et al. Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J. Clin. Endocrinol. Metab. 95, E352–E357 (2010).
Carpenter, T. O., Imel, E. A., Holm, I. A., Jan de Beur, S. M. & Insogna, K. L. A clinician’s guide to X-linked hypophosphatemia. J. Bone Miner. Res. 26, 1381–1388 (2011).
Linglart, A. et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr. Connect. 3, R13–R30 (2014).
Gizard, A. et al. Outcomes of orthopedic surgery in a cohort of 49 patients with X-linked hypophosphatemic rickets (XLHR). Endocr. Connect. 6, 566–573 (2017).
Kocaoglu, M., Bilen, F. E., Sen, C., Eralp, L. & Balci, H. I. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J. Bone Joint Surg. Br. 93, 52–56 (2011).
Matsubara, H. et al. Deformity correction for vitamin D-resistant hypophosphatemic rickets of adults. Arch. Orthop. Trauma Surg. 128, 1137–1143 (2008).
Sharkey, M. S., Grunseich, K. & Carpenter, T. O. Contemporary medical and surgical management of X-linked hypophosphatemic rickets. J. Am. Acad. Orthop. Surg. 23, 433–442 (2015).
Imel, E. A., Gray, A. K., Padgett, L. R. & Econs, M. J. Iron and fibroblast growth factor 23 in X-linked hypophosphatemia. Bone 60, 87–92 (2014).
Endo, I. et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone 42, 1235–1239 (2008).
Jonsson, K. B. et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348, 1656–1663 (2003).
Beck-Nielsen, S. S. et al. Phenotype presentation of hypophosphatemic rickets in adults. Calcif. Tissue Int. 87, 108–119 (2010).
Che, H. et al. Impaired quality of life in adults with X-linked hypophosphatemia and skeletal symptoms. Eur. J. Endocrinol. 174, 325–333 (2016).
Biosse Duplan, M. et al. Phosphate and vitamin D prevent periodontitis in X-linked hypophosphatemia. J. Dent. Res. 96, 388–395 (2017).
Carpenter, T. O. et al. Burosumab therapy in children with X-linked hypophosphatemia. N. Engl. J. Med. 378, 1987–1998 (2018).
Carpenter, T. O. et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Invest. 124, 1587–1597 (2014).
Aono, Y. et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J. Bone Miner. Res. 24, 1879–1888 (2009).
Ultragenyx Pharmaceuticals. Presentations & publications — rare disease drug development. Ultragenyx http://www.ultragenyx.com/pipeline/publications (2018).
European Medicines Agency. New medicine for rare bone disease. EMA https://www.ema.europa.eu/en/news/new-medicine-rare-bone-disease (2017).
US Food & Drug Administration. FDA approves first therapy for rare inherited form of rickets, x-linked hypophosphatemia. FDA.gov https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm604810.htm (2018).
Insogna, K. L. et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J. Bone Miner. Res. 33, 1383–1393 (2018).
Connor, J. et al. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J. Clin. Endocrinol. Metab. 100, 3625–3632 (2015).
Chen, Y. et al. A reporting tool for practice guidelines in health care: the RIGHT statement. Ann. Intern. Med. 166, 128–132 (2017).
Guyatt, G. H. et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 64, 395–400 (2011).
American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics 114, 874–877 (2004).
Flynn, J. T. et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140, e20171904 (2017).
Gaucher, C. et al. PHEX analysis in 118 pedigrees reveals new genetic clues in hypophosphatemic rickets. Hum. Genet. 125, 401–411 (2009).
Mäkitie, O. et al. Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 88, 3591–3597 (2003).
Holm, I. A., Huang, X. & Kunkel, L. M. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am. J. Hum. Genet. 60, 790–797 (1997).
Holm, I. A. et al. Mutational analysis and genotype-phenotype correlation of the PHEX gene in X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 86, 3889–3899 (2001).
Francis, F. et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X–linked hypophosphatemic rickets. Nat. Genet. 11, 130–136 (1995).
Dixon, P. H. et al. Mutational analysis of PHEX gene in X-linked hypophosphatemia. J. Clin. Endocrinol. Metab.83, 3615–3623 (1998).
Tyynismaa, H., Kaitila, I., Näntö-Salonen, K., Ala-Houhala, M. & Alitalo, T. Identification of fifteen novel PHEX gene mutations in Finnish patients with hypophosphatemic rickets. Hum. Mutat. 15, 383–384 (2000).
Ichikawa, S. et al. Mutational survey of the PHEX gene in patients with X-linked hypophosphatemic rickets. Bone 43, 663–666 (2008).
Ruppe, M. D. et al. Mutational analysis of PHEX, FGF23 and DMP1 in a cohort of patients with hypophosphatemic rickets. Clin. Endocrinol. 74, 312–318 (2011).
Morey, M. et al. Genetic diagnosis of X-linked dominant hypophosphatemic rickets in a cohort study: Tubular reabsorption of phosphate and 1,25(OH) 2 D serum levels are associated with PHEX mutation type. BMC Med. Genet. 12, 116 (2011).
Kinoshita, Y. et al. Mutational analysis of patients with FGF23-related hypophosphatemic rickets. Eur. J. Endocrinol. 167, 165–172 (2012).
Guven, A. et al. Mutational analysis of PHEX, FGF23 and CLCN5 in patients with hypophosphataemic rickets. Clin. Endocrinol. 87, 103–112 (2017).
Beck-Nielsen, S. S., Brixen, K., Gram, J. & Brusgaard, K. Mutational analysis of PHEX, FGF23, DMP1, SLC34A3 and CLCN5 in patients with hypophosphatemic rickets. J. Hum. Genet. 57, 453–458 (2012).
Goji, K., Ozaki, K., Sadewa, A. H., Nishio, H. & Matsuo, M. Somatic and germline mosaicism for a mutation of the PHEX gene can lead to genetic transmission of X-linked hypophosphatemic rickets that mimics an autosomal dominant trait. J. Clin. Endocrinol. Metab. 91, 365–370 (2006).
Christie, P. T., Harding, B., Nesbit, M. A., Whyte, M. P. & Thakker, R. V. X-Linked hypophosphatemia attributable to pseudoexons of the PHEX gene. J. Clin. Endocrinol. Metab. 86, 3840–3844 (2001).
Abe, K., Ooshima, T., Lily, T. S., Yasufuku, Y. & Sobue, S. Structural deformities of deciduous teeth in patients with hypophosphatemic vitamin D-resistant rickets. Oral Surg. Oral Med. Oral Pathol. 65, 191–198 (1988).
Chaussain-Miller, C. et al. Dentin structure in familial hypophosphatemic rickets: benefits of vitamin D and phosphate treatment. Oral Dis. 13, 482–489 (2007).
Verge, C. F. et al. Effects of therapy in X-linked hypophosphatemic rickets. N. Engl. J. Med. 325, 1843–1848 (1991).
Zivicnjak, M. et al. Age-related stature and linear body segments in children with X-linked hypophosphatemic rickets. Pediatr. Nephrol. 26, 223–231 (2011).
Sochett, E. et al. Growth and metabolic control during puberty in girls with X-linked hypophosphataemic rickets. Horm. Res. 61, 252–256 (2004).
Haffner, D. & Waldegger, S. in Pediatric Kidney Disease 2nd edn Ch. 35 (eds Geary, D.-F. & Schaefer, F.) 953–972 (Springer-Verlag Berlin Heidelberg, 2016).
Quinlan, C. et al. Growth in PHEX-associated X-linked hypophosphatemic rickets: the importance of early treatment. Pediatr. Nephrol. 27, 581–588 (2012).
Carlsen, N. L., Krasilnikoff, P. A. & Eiken, M. Premature cranial synostosis in X-linked hypophosphatemic rickets: possible precipitation by 1-alpha-OH-cholecalciferol intoxication. Acta Paediatr. Scand. 73, 149–154 (1984).
Vega, R. A. et al. Hypophosphatemic rickets and craniosynostosis: a multicenter case series. J. Neurosurg. Pediatr. 17, 694–700 (2016).
Chesher, D. et al. Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J. Inherit. Metab. Dis. 41, 865–976 (2018).
Berndt, M. et al. Clinical course of hypophosphatemic rickets in 23 adults. Clin. Nephrol. 45, 33–41 (1996).
Penido, M. G. M. G. & Alon, U. S. Hypophosphatemic rickets due to perturbations in renal tubular function. Pediatr. Nephrol. 29, 361–373 (2014).
Carpenter, T. O. et al. Rickets. Nat. Rev. Dis. Primers 3, 17101 (2017).
Brodehl, J., Krause, A. & Hoyer, P. F. Assessment of maximal tubular phosphate reabsorption: comparison of direct measurement with the nomogram of Bijvoet. Pediatr. Nephrol. 2, 183–189 (1988).
Yamazaki, Y. et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 87, 4957–4960 (2002).
Souberbielle, J.-C. et al. Evaluation of a new fully automated assay for plasma intact FGF23. Calcif. Tissue Int. 101, 510–518 (2017).
Carpenter, T. O. et al. Effect of paricalcitol on circulating parathyroid hormone in X-linked hypophosphatemia: a randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 99, 3103–3111 (2014).
Bettinelli, A., Bianchi, M. L., Mazzucchi, E., Gandolini, G. & Appiani, A. C. Acute effects of calcitriol and phosphate salts on mineral metabolism in children with hypophosphatemic rickets. J. Pediatr. 118, 372–376 (1991).
Blydt-Hansen, T. D., Tenenhouse, H. S. & Goodyer, P. PHEX expression in parathyroid gland and parathyroid hormone dysregulation in X-linked hypophosphatemia. Pediatr. Nephrol. 13, 607–611 (1999).
Whyte, M. P., Schranck, F. W. & Armamento-Villareal, R. X-Linked hypophosphatemia: a search for gender, race, anticipation, or parent of origin effects on disease expression in children. J. Clin. Endocrinol. Metab. 81, 4075–4080 (1996).
Tiosano, D. & Hochberg, Z. Hypophosphatemia: the common denominator of all rickets. J. Bone Miner. Metab. 27, 392–401 (2009).
Goldsweig, B. K. & Carpenter, T. O. Hypophosphatemic rickets: lessons from disrupted FGF23 control of phosphorus homeostasis. Curr. Osteoporos. Rep. 13, 88–97 (2015).
Wagner, C. A., Rubio-Aliaga, I., Biber, J. & Hernando, N. Genetic diseases of renal phosphate handling. Nephrol. Dial. Transplant. 29 (Suppl. 4), 45–54 (2014).
Ma, S. L. et al. Whole exome sequencing reveals novel PHEX splice site mutations in patients with hypophosphatemic rickets. PLOS ONE 10, e0130729 (2015).
Yuan, L. et al. Identification of a novel PHEX mutation in a Chinese family with X-linked hypophosphatemic rickets using exome sequencing. Biol. Chem. 396, 27–33 (2015).
Lal, D. et al. Increased probability of co-occurrence of two rare diseases in consanguineous families and resolution of a complex phenotype by next generation sequencing. PLOS ONE 11, e0146040 (2016).
Yeo, A., James, K. & Ramachandran, M. Normal lower limb variants in children. BMJ 350, h3394 (2015).
Sass, P. & Hassan, G. Lower extremity abnormalities in children. Am. Fam. Physician 68, 461–468 (2003).
Miyamoto, J., Koto, S. & Hasegawa, Y. Final height of Japanese patients with X-linked hypophosphatemic rickets: effect of vitamin D and phosphate therapy. Endocr. J. 47, 163–167 (2000).
Balsan, S. & Tieder, M. Linear growth in patients with hypophosphatemic vitamin D-resistant rickets: influence of treatment regimen and parental height. J. Pediatr. 116, 365–371 (1990).
Fuente, R. et al. X-Linked hypophosphatemia and growth. Rev. Endocr. Metab. Disord. 18, 107–115 (2017).
Saraff, V. et al. Sex-, age-, and height-specific reference curves for the 6-min walk test in healthy children and adolescents. Eur. J. Pediatr. 174, 837–840 (2015).
Murthy, A. S. X-linked hypophosphatemic rickets and craniosynostosis. J. Craniofac. Surg. 20, 439–442 (2009).
Rothenbuhler, A. et al. High incidence of cranial synostosis and Chiari I malformation in children with X-linked hypophosphatemic rickets (XLHR). J. Bone Miner. Res. 34, 490–496 (2018).
Ros, I. et al. Hypophosphatemic osteomalacia: a report of five cases and evaluation of bone markers. J. Bone Miner. Metab. 23, 266–269 (2005).
Tsuru, N., Chan, J. C. & Chinchilli, V. M. Renal hypophosphatemic rickets. Growth and mineral metabolism after treatment with calcitriol (1,25-dihydroxyvitamin D3) and phosphate supplementation. Am. J. Child 141, 108–110 (1987).
Costa, T. et al. X-Linked hypophosphatemia: effect of calcitriol on renal handling of phosphate, serum phosphate, and bone mineralization. J. Clin. Endocrinol. Metab. 52, 463–472 (1981).
Chesney, R. W. et al. Long-term influence of calcitriol (1,25-dihydroxyvitamin D) and supplemental phosphate in X-linked hypophosphatemic rickets. Pediatrics 71, 559–567 (1983).
Magnusson, P. et al. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 60, 257–265 (2001).
Rasmussen, H. et al. Long-term treatment of familial hypophosphatemic rickets with oral phosphate and 1 alpha-hydroxyvitamin D3. J. Pediatr. 99, 16–25 (1981).
Alon, U., Lovell, H. B. & Donaldson, D. L. Nephrocalcinosis, hyperparathyroidism, and renal failure in familial hypophosphatemic rickets. Clin. Pediatr. 31, 180–183 (1992).
Makitie, O., Kooh, S. W. & Sochett, E. Prolonged high-dose phosphate treatment: a risk factor for tertiary hyperparathyroidism in X-linked hypophosphatemic rickets. Clin. Endocrinol. 58, 163–168 (2003).
Schmitt, C. P. & Mehls, O. The enigma of hyperparathyroidism in hypophosphatemic rickets. Pediatr. Nephrol. 19, 473–477 (2004).
Igaki, J. M. et al. High iFGF23 level despite hypophosphatemia is one of the clinical indicators to make diagnosis of XLH. Endocr. J. 58, 647–655 (2011).
Kubota, T. et al. Serum fibroblast growth factor 23 is a useful marker to distinguish vitamin D-deficient rickets from hypophosphatemic rickets. Horm. Res. Paediatr. 81, 251–257 (2014).
Ruppe, M. D. et al. Effect of four monthly doses of a human monoclonal anti-FGF23 antibody (KRN23) on quality of life in X-linked hypophosphatemia. Bone Rep. 5, 158–162 (2016).
Zhang, X. et al. Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J. Clin. Pharmacol. 56, 176–185 (2016).
Zhang, X. et al. Population pharmacokinetic and pharmacodynamic analyses from a 4-month intradose escalation and its subsequent 12-month dose titration studies for a human monoclonal anti-FGF23 antibody (KRN23) in adults with X-linked hypophosphatemia. J. Clin. Pharmacol. 56, 429–438 (2016).
Liang, G., Katz, L. D., Insogna, K. L., Carpenter, T. O. & Macica, C. M. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif. Tissue Int. 85, 235–246 (2009).
Melhem, E., Assi, A., El Rachkidi, R. & Ghanem, I. EOS(®) biplanar X-ray imaging: concept, developments, benefits, and limitations. J. Child. Orthop. 10, 1–14 (2016).
Goodyer, P. R., Kronick, J. B., Jequier, S., Reade, T. M. & Scriver, C. R. Nephrocalcinosis and its relationship to treatment of hereditary rickets. J. Pediatr. 111, 700–704 (1987).
Keskin, M., Savas-Erdeve, S., Sagsak, E., Çetinkaya, S. & Aycan, Z. Risk factors affecting the development of nephrocalcinosis, the most common complication of hypophosphatemic rickets. J. Pediatr. Endocrinol. Metab. 28, 1333–1337 (2015).
Seikaly, M. G. & Baum, M. Thiazide diuretics arrest the progression of nephrocalcinosis in children with X-linked hypophosphatemia. Pediatrics 108, E6 (2001).
Eddy, M. C., McAlister, W. H. & Whyte, M. P. X-Linked hypophosphatemia: normal renal function despite medullary nephrocalcinosis 25 years after transient vitamin D2-induced renal azotemia. Bone 21, 515–520 (1997).
Dremmen, M. H. G. et al. Does the addition of a ‘black bone’ sequence to a fast multisequence trauma MR protocol allow MRI to replace CT after traumatic brain injury in children? Am. J. Neuroradiol. 38, 2187–2192 (2017).
Cheung, M. et al. Cortical and trabecular bone density in X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 98, E954–E961 (2013).
Veilleux, L. N., Cheung, M. S., Glorieux, F. H. & Rauch, F. The muscle-bone relationship in X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 98, E990–E995 (2013).
Beck-Nielsen, S. S., Brixen, K., Gram, J. & Mølgaard, C. High bone mineral apparent density in children with X-linked hypophosphatemia. Osteoporos. Int. 24, 2215–2221 (2013).
Alon, U. S. et al. Hypertension in hypophosphatemic rickets—role of secondary hyperparathyroidism. Pediatr. Nephrol. 18, 155–158 (2003).
Nakamura, Y., Takagi, M., Takeda, R., Miyai, K. & Hasegawa, Y. Hypertension is a characteristic complication of X-linked hypophosphatemia. Endocr. J. 64, 283–289 (2017).
Takashi, Y. et al. Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr. Res. 42, 132–137 (2017).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Vered, I., Vered, Z., Perez, J. E., Jaffe, A. S. & Whyte, M. P. Normal left ventricular performance in children with X-linked hypophosphatemic rickets: a Doppler echocardiography study. J. Bone Miner. Res. 5, 469–474 (1990).
Nehgme, R., Fahey, J. T., Smith, C. & Carpenter, T. O. Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J. Clin. Endocrinol. Metab. 82, 2450–2454 (1997).
Moltz, K. C., Friedman, A. H., Nehgme, R. A., Kleinman, C. S. & Carpenter, T. O. Ectopic cardiac calcification associated with hyperparathyroidism in a boy with hypophosphatemic rickets. Curr. Opin. Pediatr. 13, 373–375 (2001).
Harrell, R. M., Lyles, K. W., Harrelson, J. M., Friedman, N. E. & Drezner, M. K. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia. Induction and maintenance with phosphorus and calcitriol. J. Clin. Invest. 75, 1858–1868 (1985).
Ariceta, G. & Langman, C. B. Growth in X-linked hypophosphatemic rickets. Eur. J. Pediatr. 166, 303–309 (2007).
Econs, M. J. Conventional therapy in adults with XLH improves dental manifestations, but not enthesopathy. J. Clin. Endocrinol. Metab. 100, 3622–3624 (2015).
Chaussain-Miller, C. et al. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets: prevention by early treatment with 1-hydroxyvitamin D. J. Pediatr. 142, 324–331 (2003).
Baroncelli, G. I. et al. Prevalence and pathogenesis of dental and periodontal lesions in children with X-linked hypophosphatemic rickets. Eur. J. Paediatr. Dent. 7, 61–66 (2006).
Lempicki, M. et al. Magnetic resonance imaging features as surrogate markers of X-linked hypophosphatemic rickets activity. Horm. Res. Paediatr. 87, 244–253 (2017).
Glorieux, F. H., Marie, P. J., Pettifor, J. M. & Delvin, E. E. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N. Engl. J. Med. 303, 1023–1031 (1980).
Seikaly, M., Browne, R. & Baum, M. Nephrocalcinosis is associated with renal tubular acidosis in children with X-linked hypophosphatemia. Pediatrics 97, 91–93 (1996).
Kiattisunthorn, K., Wutyam, K., Indranoi, A. & Vasuvattakul, S. Randomized trial comparing pulse calcitriol and alfacalcidol for the treatment of secondary hyperparathyroidism in haemodialysis patients. Nephrology 16, 277–284 (2011).
Shroff, R. et al. Clinical practice recommendations for treatment with active vitamin D analogues in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol. Dial. Transplant. 32, 1114–1127 (2017).
Carpenter, T. O. et al. 24,25 Dihydroxyvitamin D supplementation corrects hyperparathyroidism and improves skeletal abnormalities in X-linked hypophosphatemic rickets—a clinical research center study. J. Clin. Endocrinol. Metab. 81, 2381–2388 (1996).
Sullivan, W., Carpenter, T., Glorieux, F., Travers, R. & Insogna, K. A prospective trial of phosphate and 1,25-dihydroxyvitamin D3 therapy in symptomatic adults with X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 75, 879–885 (1992).
Oliveri, M. B., Cassinelli, H., Bergadá, C. & Mautalen, C. A. Bone mineral density of the spine and radius shaft in children with X-linked hypophosphatemic rickets (XLH). Bone Miner. 12, 91–100 (1991).
Reid, I. R. et al. X-Linked hypophosphatemia: skeletal mass in adults assessed by histomorphometry, computed tomography, and absorptiometry. Am. J. Med. 90, 63–69 (1991).
Vaisbich, M. H. & Koch, V. H. Hypophosphatemic rickets: results of a long-term follow-up. Pediatr. Nephrol. 21, 230–234 (2006).
Yavropoulou, M. P. et al. Cinacalcet in hyperparathyroidism secondary to X-linked hypophosphatemic rickets: case report and brief literature review. Hormones 9, 274–278 (2010).
Vaughn, L. K., Meyer, R. A. & Meyer, M. H. Increased metabolic rate in X-linked hypophosphatemic mice. Endocrinology 118, 441–445 (1986).
Angelin, B., Larsson, T. E. & Rudling, M. Circulating fibroblast growth factors as metabolic regulators — a critical appraisal. Cell Metab. 16, 693–705 (2012).
Ræder, H., Shaw, N., Netelenbos, C. & Bjerknes, R. A case of X-linked hypophosphatemic rickets: complications and the therapeutic use of cinacalcet. Eur. J. Endocrinol. 159, S101–S105 (2008).
Alon, U. S. et al. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin. J. Am. Soc. Nephrol. 3, 658–664 (2008).
Warady, B. A. et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet in pediatric patients with chronic kidney disease and secondary hyperparathyroidism receiving dialysis. Pediatr. Nephrol. 34, 475–486 (2019).
US Food & Drug Administration. CRYSVITA (prescribing information). FDA.gov https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761068s000lbl.pdf (2018).
European Medicines Agency. Crysvita. Annex I — summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/crysvita-epar-product-information_en.pdf (2018).
Collins, M. Burosumab: at long last, an effective treatment for FGF23-associated hypophosphatemia. J. Bone Miner. Res. 33, 1381–1382 (2018).
Emma, F. & Haffner, D. FGF23 blockade coming to clinical practice. Kidney Int. 94, 846–848 (2018).
Capelli, S. et al. Clinical and molecular heterogeneity in a large series of patients with hypophosphatemic rickets. Bone 79, 143–149 (2015).
Dudkiewicz, I., Schindler, A. & Ganel, A. Elongation of long bones for short stature in patients with hypophosphatemic rickets. Isr. Med. Assoc. J. 5, 66–67 (2003).
Borghi, M. M. S., Coates, V. & Omar, H. A. Evaluation of stature development during childhood and adolescence in individuals with familial hypophosphatemic rickets. ScientificWorldJournal 5, 868–873 (2005).
Seikaly, M. G., Browne, R. H. & Baum, M. The effect of phosphate supplementation on linear growth in children with X-linked hypophosphatemia. Pediatrics 94, 478–481 (1994).
Rothenbuhler, A. et al. Two-year recombinant human growth hormone (rhGH) treatment is more effective in pre-pubertal compared to pubertal short children with X-linked hypophosphatemic rickets (XLHR). Growth Horm. IGF Res. 36, 11–15 (2017).
Živicnjak, M. et al. Three-year growth hormone treatment in short children with X-linked hypophosphatemic rickets: effects on linear growth and body disproportion. J. Clin. Endocrinol. Metab. 96, E2097–E2105 (2011).
Meyerhoff, N. et al. Effects of growth hormone treatment on adult height in severely short children with X-linked hypophosphatemic rickets. Pediatr. Nephrol. 33, 447–456 (2018).
Baroncelli, G. I., Bertelloni, S., Ceccarelli, C. & Saggese, G. Effect of growth hormone treatment on final height, phosphate metabolism, and bone mineral density in children with X-linked hypophosphatemic rickets. J. Pediatr. 138, 236–243 (2001).
Bhambri, R. et al. Changes in bone mineral density following treatment of osteomalacia. J. Clin. Densitom. 9, 120–127 (2006).
Teitelbaum, S. L., Rosenberg, E. M., Bates, M. & Avioli, L. V. The effects of phosphate and vitamin D therapy on osteopenic, hypophosphatemic osteomalacia of childhood. Clin. Orthop. Relat. Res. 116, 38–47 (1976).
Song, S.-H. et al. The significance of serum phosphate level on healing index and its relative effects in skeletally immature and mature patients with hypophosphatemic rickets. BioMed Res. Int. 2014, 569530 (2014).
Nagata, Y. et al. Evaluation of bone markers in hypophosphatemic rickets/osteomalacia. Endocrine 40, 315–317 (2011).
Alon, U. & Chan, J. C. Effects of hydrochlorothiazide and amiloride in renal hypophosphatemic rickets. Pediatrics 75, 754–763 (1985).
Kovacs, C. S. & Kronenberg, H. M. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 18, 832–872 (1997).
Chinoy, M. A., Javed, M. I., Khan, A. & Sadruddin, N. Alkaline phosphatase as a screening test for osteomalacia. J. Ayub Med. Coll. Abbottabad 23, 23–25 (2011).
Jacobson, J. A. & Kalume-Brigido, M. Case 97: X-linked hypophosphatemic osteomalacia with insufficiency fracture. Radiology 240, 607–610 (2006).
Suzuki, E. et al. Patients with hypophosphatemic osteomalacia need continuous treatment during adulthood. Clin. Pediatr. Endocrinol. 18, 29–33 (2009).
Veilleux, L.-N., Cheung, M., Ben Amor, M. & Rauch, F. Abnormalities in muscle density and muscle function in hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 97, E1492–E1498 (2012).
Makras, P., Hamdy, N. A. T., Kant, S. G. & Papapoulos, S. E. Normal growth and muscle dysfunction in X-linked hypophosphatemic rickets associated with a novel mutation in the PHEX gene. J. Clin. Endocrinol. Metab. 93, 1386–1389 (2008).
Gjørup, H., Kjaer, I., Beck-Nielsen, S. S., Poulsen, M. R. & Haubek, D. A radiological study on intra- and extra-cranial calcifications in adults with X-linked hypophosphatemia and associations with other mineralizing enthesopathies and childhood medical treatment. Orthod. Craniofac. Res. 19, 114–125 (2016).
Polisson, R. P. et al. Calcification of entheses associated with X-linked hypophosphatemic osteomalacia. N. Engl. J. Med. 313, 1–6 (1985).
Petje, G., Meizer, R., Radler, C., Aigner, N. & Grill, F. Deformity correction in children with hereditary hypophosphatemic rickets. Clin. Orthop. Relat. Res. 466, 3078–3085 (2008).
Rohmiller, M. T., Tylkowski, C., Kriss, V. M. & Mier, R. J. The effect of osteotomy on bowing and height in children with X-linked hypophosphatemia. J. Pediatr. Orthop. 19, 114–118 (1999).
Nielsen, L. H., Rahbek, E. T., Beck-Nielsen, S. S. & Christesen, H. T. Treatment of hypophosphataemic rickets in children remains a challenge. Dan. Med. J. 61, A4874 (2014).
Horn, A., Wright, J., Bockenhauer, D., Van’t Hoff, W. & Eastwood, D. M. The orthopaedic management of lower limb deformity in hypophosphataemic rickets. J. Child. Orthop. 11, 298–305 (2017).
Evans, G. A., Arulanantham, K. & Gage, J. R. Primary hypophosphatemic rickets. Effect of oral phosphate and vitamin D on growth and surgical treatment. J. Bone Joint Surg. Am. 62, 1130–1138 (1980).
Ferris, B., Walker, C., Jackson, A. & Kirwan, E. The orthopaedic management of hypophosphataemic rickets. J. Pediatr. Orthop. 11, 367–373 (1991).
Rubinovitch, M., Said, S. E., Glorieux, F. H., Cruess, R. L. & Rogala, E. Principles and results of corrective lower limb osteotomies for patients with vitamin D-resistant hypophosphatemic rickets. Clin. Orthop. Relat. Res. 237, 264–270 (1988).
Stanitski, D. F. Treatment of deformity secondary to metabolic bone disease with the Ilizarov technique. Clin. Orthop. Relat. Res. 301, 38–41 (1994).
Danino, B. et al. Guided growth: preliminary results of a multinational study of 967 physes in 537 patients. J. Child. Orthop. 12, 91–96 (2018).
Popkov, A., Aranovich, A. & Popkov, D. Results of deformity correction in children with X-linked hereditary hypophosphatemic rickets by external fixation or combined technique. Int. Orthop. 39, 2423–2431 (2015).
Eralp, L., Kocaoglu, M., Toker, B., Balcı, H. I. & Awad, A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch. Orthop. Trauma Surg. 131, 581–589 (2011).
Stevens, P. M. & Klatt, J. B. Guided growth for pathological physes: radiographic improvement during realignment. J. Pediatr. Orthop. 28, 632–639 (2008).
Khan, F. A. et al. Effect of local alignment on compartmental patterns of knee osteoarthritis. J. Bone Joint Surg. Am. 90, 1961–1969 (2008).
World Health Organization. International classification of functioning, disability and health (ICF). WHO http://www.who.int/classifications/icf/en/ (2018).
Ye, L., Liu, R., White, N., Alon, U. S. & Cobb, C. M. Periodontal status of patients with hypophosphatemic rickets: a case series. J. Periodontol. 82, 1530–1535 (2011).
Opsahl Vital, S. et al. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone 50, 989–997 (2012).
Beltes, C. & Zachou, E. Endodontic management in a patient with vitamin D–resistant rickets. J. Endod. 38, 255–258 (2012).
Douyere, D., Joseph, C., Gaucher, C., Chaussain, C. & Courson, F. Familial hypophosphatemic vitamin D-resistant rickets—prevention of spontaneous dental abscesses on primary teeth: a case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 107, 525–530 (2009).
Kawakami, M. & Takano-Yamamoto, T. Orthodontic treatment of a patient with hypophosphatemic vitamin D-resistant rickets. ASDC J. Dent. Child 64, 395–399 (1997).
Seow, W. K., Brown, J. P., Tudehope, D. A. & O’Callaghan, M. Dental defects in the deciduous dentition of premature infants with low birth weight and neonatal rickets. Pediatr. Dent. 6, 88–92 (1984).
Al-Jundi, S. H., Hammad, M. M. & Dabous, I. Case report: hypophosphatemic rickets and aggressive periodontitis: a review of the role of dentine matrix protein 1 in the pathogenesis. Eur. Arch. Paediatr. Dent. 12, 46–50 (2011).
Resnick, D. Implant placement and guided tissue regeneration in a patient with congenital vitamin D-resistant rickets. J. Oral Implantol. 24, 214–218 (1998).
Friberg, B. Brånemark system implants and rare disorders: a report of six cases. Int. J. Periodontics Restorative Dent. 33, 139–148 (2013).
Bergendal, B. & Ljunggren, Ö. Treatment with oral implants in x-linked hypophosphatemic rickets and hypophosphatasia: report from a workshop. Swed. Dent. J. 25, 165 (2001).
Davies, M., Kane, R. & Valentine, J. Impaired hearing in X-linked hypophosphataemic (vitamin-D-resistant) osteomalacia. Ann. Intern. Med. 100, 230–232 (1984).
O’Malley, S. P., Adams, J. E., Davies, M. & Ramsden, R. T. The petrous temporal bone and deafness in X-linked hypophosphataemic osteomalacia. Clin. Radiol. 39, 528–530 (1988).
Currarino, G. Sagittal synostosis in X-linked hypophosphatemic rickets and related diseases. Pediatr. Radiol. 37, 805–812 (2007).
Willis, F. R. & Beattie, T. J. Craniosynostosis in X-linked hypophosphataemic rickets. J. Paediatr. Child Health 33, 78–79 (1997).
Glass, L. R. D., Dagi, T. F. & Dagi, L. R. Papilledema in the setting of x-linked hypophosphatemic rickets with craniosynostosis. Case Rep. Ophthalmol. 2, 376–381 (2011).
Caldemeyer, K. S. et al. Chiari I malformation: association with hypophosphatemic rickets and MR imaging appearance. Radiology 195, 733–738 (1995).
Zelenchuk, L. V., Hedge, A.-M. & Rowe, P. S. N. PHEX mimetic (SPR4-peptide) corrects and improves HYP and wild type mice energy-metabolism. PLOS ONE 9, e97326 (2014).
Rowe, P. S. N. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit. Rev. Eukaryot. Gene Expr. 22, 61–86 (2012).
Pronicka, E. et al. Anthropometric characteristics of X-linked hypophosphatemia. Am. J. Med. Genet. 126A, 141–149 (2004).
Wohrle, S. et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 28, 899–911 (2013).
Yuan, B. et al. Hexa-D-arginine treatment increases 7B2*PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. J. Bone Miner. Res. 28, 56–72 (2013).
Johnson, K. et al. Therapeutic effects of FGF23 c-tail Fc in a murine preclinical model of X-linked hypophosphatemia via the selective modulation of phosphate reabsorption. J. Bone Miner. Res. 32, 2062–2073 (2017).
Carpenter, T. O. The expanding family of hypophosphatemic syndromes. J. Bone Miner. Metab. 30, 1–9 (2012).
Holland, M. R. Renal tubular reabsorption of phosphate calculation. BasPath.co.uk http://www.baspath.co.uk/calculations/renal_tubular_reabsorption_of_ph.htm (2014).
Mian, A. N. & Schwartz, G. J. Measurement and estimation of glomerular filtration rate in children. Adv. Chronic Kidney Dis. 24, 348–356 (2017).
Soares, A. A. et al. Glomerular filtration rate measurement and prediction equations. Clin. Chem. Lab. Med. 47, 1023–1032 (2009).
Acknowledgements
The authors thank the European Society for Paediatric Nephrology (ESPN) for launching, organizing and funding this initiative, which included travel and housing costs for the core group members. The funder had no influence on the content of the guideline. The authors also acknowledge the valuable contributions of the expert panel who participated in the Delphi technique. The expert panel included N. Gittoes, University Hospitals Birmingham, BHP, Birmingham, UK; C. Di Somma, AOU Federico II University, Naples, Italy; N. Appelman-Dijkstra, Leiden University Medical Center, Leiden, Netherlands; S. Mora, IRCCS San Raffele Scientific Institute, Milano, Italy; C. Grasemann, University Hospital Essen, Pediatric Endocrinology, Essen, Germany; C. P. Schmitt, Centre of Pediatric and Adolescent Medicine Heidelberg, Heidelberg, Germany; C. Hofmann, Pediatric Hospital, Pediatric Rheumatology and Osteology, University of Würzburg, Würzburg, Germany; T. J. Neuhaus, Children’s Hospital Lucerne, Lucerne, Switzerland; S. Faisal Ahmed, Developmental Endocrinology Research Group, Royal Hospital for Children, University of Glasgow, Glasgow, UK; S. Turan, Marmara University, School of Medicine, Istanbul, Turkey; R. Ramakrishnan, Alder Hey Children’s NHS Foundation Trust, Liverpool, UK; J. H. Davies, University Hospital Southampton, Southampton, UK; G. A. Martos-Moreno, Department of Endocrinology, Hospital Infantil, Univarsitario Nino Jesus, Madrid, Spain; D. de Sotto-Esteban, Clinica Rotger, Balearic Islands, Spain; A. Bereket, Marmara University Hospital, Istanbul, Turkey; G. Weber, San Raffaele Hospital, Milan, Italy; M. Kostik, Saint-Petersburg State Pediatric Medical University, St Petersburg, Russia; A. Doulgeraki, Department of Bone and Mineral Metabolism, Institute of Child Health, Athens, Greece; F. Courson, Hospital Bretonneau, Paris, France; A. Raimann, Medical University of Vienna, Vienna, Austria; G. Häusler, Medical University of Vienna, Vienna, Austria; S. Sparre Beck-Nielsen, Lillebaelt Hospital, Kolding, Denmark; P. Kotnik, University Medical Centre Ljubljana, Ljubljana, Slovenia; R. Padidela, Royal Manchester Children’s Hospital, Manchester, UK; S. Stabouli, Aristotle University Thessaloniki, Hippocratio Hospital, Thessaloniki, Greece; C. Eller-Vainicher, Fondazione Ca’Granda IRCCS Ospedale Maggiore Policlinico, Milan, Italy; N. Shaw, Birmingham Children’s Hospital, Birmingham, UK; V. Zhukouskaya, Medicine and Surgery, Division of Endocrinology, University of Naples Federico II, Naples, Italy; V. Saraff, Birmingham Women’s and Childen’s Hospital, Birmingham, UK; Z. Sumnik, Motol University Hospital, Prague, Czech Republic; G. Ariceta, Hospital Universitari Vall d’ Hebron, Barcelona, Spain; A. Rothenbuhler, Reference Center for Rare Disorders of Calcium and Phosphate Metabolism, APHP, Paris, France; C. Jefferies, Starship Children’s Health, Auckland, New Zealand; J.-P. Salles, Hôpital des Enfants CHU de Toulouse, Toulouse, France; C. Zillikens, Erasmus MC Rotterdam, Rotterdam, Netherlands; C. P. Burren, University Hospitals Bristol NHS Foundation Trust, Bristol, UK; S. Thiele, University of Luebeck, Luebeck, Germany; E. Emese Boros, HUDERF, Brussels, Belgium; L, Castano, Cruces University Hospital, Bilbao, Spain; N. Bishop, Sheffield Children’s Hospital, Sheffield, UK; J. Nevoux, ENT Department, Bicêtre Hospital, APHP, Paris, France; and G. Baroncelli, University Hospital ‘S. Chiara’, Pisa, Italy.
Reviewer information
Nature Reviews Nephrology thanks W. Hogler, T. Weber and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
D.H., F.E., D.E., M.B.D., J.B., D.S., P.W., F.D.R., C.C., P.K., L.R. and A.L. researched data for the article, made a substantial contribution to discussion of content, wrote, reviewed and edited the manuscript before submission. D.B., F.S., E.L., L.S., and K.B. made a substantial contribution to discussion of content, wrote, reviewed and edited the manuscript before submission. P.H., M.K. and M.L.B. made a substantial contribution to discussion of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.B. receives support for research and consultancy from Kyowa Kirin. A.L. receives research support from Kyowa Kirin. D.H. receives a research grant and speaker and consultant fees from Kyowa Kirin. F.E., E.L., P.K., K.B., D.S. and K.B. receive consultation fees from Kyowa Kirin. All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Osteomalacia
-
Mineralization defect of bone (soft bone) resulting in bone pain and deformations.
- Odontomalacia
-
Mineralization defect of odontoblasts resulting in soft teeth.
- Pseudofractures
-
Atraumatic lucencies (pale area revealed in radiography) extending across one cortex; by contrast, fractures are defined as lucencies extending across both cortices.
- Varus deformity
-
The distal part of the leg is deviated inwards, in relation to the femur, resulting in a bow-legged appearance.
- Valgus deformity
-
The distal part of the leg below the knee is deviated outwards, in relation to the femur, resulting in a knock-kneed appearance.
- Dolichocephaly
-
A condition in which the head is longer than would be expected relative to its width.
- Rachitic rosary
-
The prominent knobs of bone at the costochondral joints in patients with rickets (also known as beading of the ribs); the knobs create the appearance of large beads under the skin of the rib cage, hence the name by analogy with the beads of a Catholic Christian rosary.
- Harrison’s groove
-
A horizontal groove along the lower border of the thorax corresponding to the costal insertion of the diaphragm (also known as Harrison’s sulcus); it appears in rickets because of defective mineralization of the bone — the diaphragm, which is always in tension, pulls the softened bone inward.
- Intercondylar and/or intermalleolar distance
-
The distance between knees (intercondylar) and ankles (intermalleolar). It is used to assess the severity of valgus or varus leg deformities. However, it varies with age and cannot replace regular orthopaedic assessment owing to the high complexity of leg deformities (for example, torsion of limbs) in many patients.
- Craniosynostosis
-
A condition in which one or more of the fibrous sutures in a very young skull prematurely fuses by turning into bone (ossification); because the skull cannot expand perpendicular to the fused suture, it compensates by growing more in the direction parallel to the closed sutures.
- Osteotomies
-
Surgical procedures in which the bone is cut, the two ends realigned to improve shape and/or length and stabilized with internal or external fixation.
- Mechanical axis
-
A line drawn from the centre of the femoral head to the centre of the ankle joint; in a patient who is standing, this line should pass through the centre of the knee. When it does not do so, the mechanical axis is deviated.
- Retrognathism
-
A type of malocclusion that refers to an abnormal posterior positioning of the maxilla (the upper fixed bone of the jaw).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haffner, D., Emma, F., Eastwood, D.M. et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 15, 435–455 (2019). https://doi.org/10.1038/s41581-019-0152-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0152-5
This article is cited by
-
Clinical, genetic profile and therapy evaluation of 11 Chinese pediatric patients with Fanconi-Bickel syndrome
Orphanet Journal of Rare Diseases (2024)
-
The differential effect of modern intravenous iron on fibroblast growth factor 23 and phosphate in non-dialysis dependent CKD – the exploratory randomized controlled double-blind ExplorIRON-CKD study
BMC Nephrology (2024)
-
Disease Manifestations and Complications in Dutch X-Linked Hypophosphatemia Patients
Calcified Tissue International (2024)
-
Epidemiological analysis to identify predictors of X-linked hypophosphatemia (XLH) diagnosis in an Italian pediatric population: the EPIX project
Endocrine (2024)
-
Successful Burosumab Treatment in an Adult Patient with X-Linked Hypophosphatemia and Chronic Kidney Disease Stage 3b
Calcified Tissue International (2024)