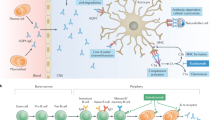
Abstract
Neuromyelitis optica spectrum disorders (NMOSD) are a type of neurological autoimmune disease characterized by attacks of CNS inflammation that are often severe and predominantly affect the spinal cord and optic nerve. The majority of individuals with NMOSD are women, many of whom are of childbearing age. Although NMOSD are rare, several small retrospective studies and case reports have indicated that pregnancy can worsen disease activity and might contribute to disease onset. NMOSD disease activity seems to negatively affect pregnancy outcomes. Moreover, some of the current NMOSD treatments are known to pose risks to the developing fetus and only limited safety data are available for others. Here, we review published studies regarding the relationship between pregnancy outcomes and NMOSD disease activity. We also assess the risks associated with using disease-modifying therapies for NMOSD during the course of pregnancy and breastfeeding. On the basis of the available evidence, we offer recommendations regarding the use of these therapies in the course of pregnancy planning in individuals with NMOSD.
Key points
-
Several studies have shown that women with neuromyelitis optica spectrum disorders (NMOSD) have an increased risk of relapse postpartum; the available data are insufficient to accurately define the relapse risk during pregnancy.
-
Aquaporin 4 (AQP4), which is the main target antigen in NMOSD, is expressed at high levels in the placenta, and women with active NMOSD have a high risk of miscarriage.
-
AQP4 antibodies cross the placenta and are found in the blood of newborn infants without causing symptoms; however, other autoantibodies or comorbid autoimmune conditions can cause symptoms in the newborn infant.
-
Azathioprine, rituximab, eculizumab and glucocorticoids seem to be relatively safe in pregnancy and are the treatments of choice; tocilizumab can be considered in women with very severe NMOSD.
-
Given the risk of obstetric complications and postpartum NMOSD relapse, patients should be informed about the advantages and risks of the use of NMOSD treatments at the time of conception or during pregnancy, and should be monitored in a multidisciplinary manner.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Jarius, S. & Wildemann, B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat. Rev. Neurol. 6, 383–392 (2010).
Jarius, S. et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat. Clin. Pract. Neurol. 4, 202–214 (2008).
Jarius, S. & Wildemann, B. The history of neuromyelitis optica. J. Neuroinflammation 10, 8 (2013).
Jarius, S. & Wildemann, B. The history of neuromyelitis optica. Part 2: ‘Spinal amaurosis’, or how it all began. J. Neuroinflammation 16, 280 (2019).
Lennon, V. A. et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004). The publication of this article led to the development of NMO-specific diagnostic criteria.
Wingerchuk, D. M. et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85, 177–189 (2015). The first set of diagnostic consensus criteria that cover both NMO and its formes frustes as well as seropositive and seronegative cases.
Borisow, N. et al. Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult. Scler. 23, 1092–1103 (2017).
Pandit, L. et al. Demographic and clinical features of neuromyelitis optica: a review. Mult. Scler. 21, 845–853 (2015).
Bourre, B. et al. Neuromyelitis optica and pregnancy. Neurology 78, 875–879 (2012).
Shimizu, Y. et al. Pregnancy-related relapse risk factors in women with anti-AQP4 antibody positivity and neuromyelitis optica spectrum disorder. Mult. Scler. 22, 1413–1420 (2016). This study showed that the relapse rate in individuals with AQP4-IgG-positive NMOSD increases during the postpartum period and, possibly, also during pregnancy.
Huang, Y. et al. Pregnancy in neuromyelitis optica spectrum disorder: a multicenter study from South China. J. Neurol. Sci. 372, 152–156 (2017).
Klawiter, E. C. et al. High risk of postpartum relapses in neuromyelitis optica spectrum disorder. Neurology 89, 2238–2244 (2017).
Tong, Y. et al. Influences of pregnancy on neuromyelitis optica spectrum disorders and multiple sclerosis. Mult. Scler. Relat. Disord. 25, 61–65 (2018).
Kim, W. et al. Influence of pregnancy on neuromyelitis optica spectrum disorder. Neurology 78, 1264–1267 (2012).
Fragoso, Y. D. et al. Neuromyelitis optica and pregnancy. J. Neurol. 260, 2614–2619 (2013).
Nour, M. M. et al. Pregnancy outcomes in aquaporin-4-positive neuromyelitis optica spectrum disorder. Neurology 86, 79–87 (2016). This study found that the risk of miscarriage is increased after onset of NMOSD.
Saadoun, S. et al. Neuromyelitis optica IgG causes placental inflammation and fetal death. J. Immunol. 191, 2999–3005 (2013). This study showed that AQP4 is expressed at high levels in the healthy placenta.
De Falco, M. et al. Down-regulation of aquaporin 4 in human placenta throughout pregnancy. In Vivo 21, 813–817 (2007).
Reuss, R. et al. A woman with acute myelopathy in pregnancy: case outcome. BMJ 339, b4026 (2009).
Jarius, S. et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J. Neuroinflammation 9, 14 (2012).
Park-Wyllie, L. et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 62, 385–392 (2000). The results of this study suggest that steroids can be teratogenic when used in the first trimester.
Hoeltzenbein, M. et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am. J. Med. Genet. A 158A, 588–596 (2012).
Hyoun, S. C., Običan, S. G. & Scialli, A. R. Teratogen update: methotrexate. Birth Defects Res. A Clin. Mol. Teratol. 94, 187–207 (2012).
Frau, J. et al. Mitoxantrone exposure in pregnancy: a new case report in a multiple sclerosis patient. Case Rep. Perinat. Med. 5, 125–126 (2016).
De Santis, M. et al. The first case of mitoxantrone exposure in early pregnancy. Neurotoxicology 28, 696–697 (2007).
Jarius, S. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J. Neuroinflammation 13, 279 (2016).
Reindl, M., Di Pauli, F., Rostásy, K. & Berger, T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat. Rev. Neurol. 9, 455–461 (2013).
Juryn´czyk, M. et al. Brain lesion distribution criteria distinguish MS from AQP4-antibody NMOSD and MOG-antibody disease. J. Neurol. Neurosurg. Psychiatry 88, 132–136 (2017).
Jarius, S. et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J. Neuroinflammation 15, 134 (2018). This article proposes a set of diagnostic criteria for MOG-IgG disease.
Trowsdale, J. & Betz, A. G. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 7, 241–246 (2006).
Clark, D. A., Chaouat, G., Wong, K., Gorczynski, R. M. & Kinsky, R. Tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens. Am. J. Reprod. Immunol. 63, 93–103 (2010).
Fu, B. et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc. Natl Acad. Sci. USA 110, E231–E240 (2013).
Tao, Y. et al. CD56brightCD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell. Mol. Immunol. 12, 77–86 (2015).
Ohkura, N., Kitagawa, Y. & Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013).
Lennon, V. A., Kryzer, T. J., Pittock, S. J., Verkman, A. S. & Hinson, S. R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005).
Varrin-Doyer, M. et al. MOG transmembrane and cytoplasmic domains contain highly stimulatory T-cell epitopes in MS. Neurol. Neuroimmunol. Neuroinflamm. 1, e20 (2014).
Davoudi, V., Keyhanian, K., Bove, R. M. & Chitnis, T. Immunology of neuromyelitis optica during pregnancy. Neurol. Neuroimmunol. Neuroinflamm. 3, e288 (2016).
Reindl, M. & Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 15, 89–102 (2019).
Jarius, S. et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain 131, 3072–3080 (2008).
Jarius, S. & Wildemann, B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol. 23, 661–683 (2013).
Jarius, S. et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflammation 13, 280 (2016). This article provides information on MOG-IgG disease activity during pregnancy.
Alves Do Rego, C. et al. Disease activity during pregnancy in patients with AQP4-Ab positive, MOG-Ab positive or double negative NMOSD [abstract P984]. Mult. Scler. 24 (Suppl. 2), 541 (2018). This study found that MOG-IgG disease attacks become less frequent during pregnancy but more frequent during the postpartum period.
Asgari, N., Henriksen, T. B., Petersen, T., Lillevang, S. T. & Weinshenker, B. G. Pregnancy outcomes in a woman with neuromyelitis optica. Neurology 83, 1576–1577 (2014).
Rubio Tabares, J. & Amaya Gonzalez, P. F. Plasma exchange therapy for a severe relapse of Devic’s disease in a pregnant woman: a case report and concise review. Clin. Neurol. Neurosurg. 148, 88–90 (2016).
Igel, C. et al. Neuromyelitis optica in pregnancy complicated by posterior reversible encephalopathy syndrome, eclampsia and fetal death. J. Clin. Med. Res. 7, 193–195 (2015).
Delgado-García, G., Chávez, Z., Rivas-Alonso, V., Corona, T. & Flores-Rivera, J. Obstetric outcomes in a Mexican cohort of patients with AQP4-antibody-seropositive neuromyelitis optica. Mult. Scler. Relat. Disord. 25, 268–270 (2018). This study identified an association between AQP4-IgG-positive NMO disease activity and poor pregnancy outcomes.
Chang, Y. et al. Study of the placentae of patients with neuromyelitis optica spectrum disorder. J. Neurol. Sci. 387, 119–123 (2018).
Chang, Y. et al. Ectrodactyly in a Chinese patient born to a mother with neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 19, 70–72 (2018).
Jakó, M. et al. The pregnancy and postnatal outcome in neuromyelitis optica: case study [abstract P03.03]. Ultrasound Obst. Gyn. 46, 128–129 (2015).
Pellkofer, H. L., Suessmair, C., Schulze, A., Hohlfeld, R. & Kuempfel, T. Course of neuromyelitis optica during inadvertent pregnancy in a patient treated with rituximab. Mult. Scler. 15, 1006–1008 (2009).
Ringelstein, M. et al. Neuromyelitis optica and pregnancy during therapeutic B cell depletion: infant exposure to anti-AQP4 antibody and prevention of rebound relapses with low-dose rituximab postpartum. Mult. Scler. 19, 1544–1547 (2013).
Jurewicz, A. & Selmaj, K. Relapse of neuromyelitis optica during pregnancy — treatment options and literature review. Clin. Neurol. Neurosurg. 130, 159–161 (2015).
Shang, W. & Liu, J. Neuromyelitis optica during pregnancy. Int. J. Gynaecol. Obstet. 115, 66–68 (2011).
Tsugawa, J., Tsuboi, Y., Inoue, H., Baba, Y. & Yamada, T. A case of anti-aquaporin 4 antibody-positive Sjogren syndrome associated with a relapsed myelitis in pregnancy [Japanese]. Rinsho Shinkeigaku 50, 27–30 (2010).
Shosha, E., Pittock, S. J., Flanagan, E. & Weinshenker, B. G. Neuromyelitis optica spectrum disorders and pregnancy: interactions and management. Mult. Scler. 23, 1808–1817 (2017).
Ajmera, M. R., Boscoe, A., Mauskopf, J., Candrilli, S. D. & Levy, M. Evaluation of comorbidities and health care resource use among patients with highly active neuromyelitis optica. J. Neurol. Sci. 384, 96–103 (2018).
Iyer, A., Elsone, L., Appleton, R. & Jacob, A. A review of the current literature and a guide to the early diagnosis of autoimmune disorders associated with neuromyelitis optica. Autoimmunity 47, 154–161 (2014).
Wingerchuk, D. M. & Weinshenker, B. G. The emerging relationship between neuromyelitis optica and systemic rheumatologic autoimmune disease. Mult. Scler. 18, 5–10 (2012).
Adawi, M., Bisharat, B. & Bowirrat, A. Systemic lupus erythematosus (SLE) complicated by neuromyelitis optica (NMO — Devic’s disease): clinic-pathological report and review of the literature. Clin. Med. Insights Case Rep. 7, 41–47 (2014).
Pittock, S. J. et al. Neuromyelitis optica and non-organ-specific autoimmunity. Arch. Neurol. 65, 78–83 (2008).
Asgari, N. et al. Aquaporin-4-autoimmunity in patients with systemic lupus erythematosus: a predominantly population-based study. Mult. Scler. 24, 331–339 (2018).
Lateef, A. & Petri, M. Systemic lupus erythematosus and pregnancy. Rheum. Dis. Clin. North Am. 43, 215–226 (2017).
Fischer-Betz, R. & Specker, C. Pregnancy in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract. Res. Clin. Rheumatol. 31, 397–414 (2017).
Lazzaroni, M. G. et al. A comprehensive review of the clinical approach to pregnancy and systemic lupus erythematosus. J. Autoimmun. 74, 106–117 (2016).
Cervera, R. Antiphospholipid syndrome. Thromb. Res. 151, S43–S47 (2017).
Long, Y. et al. Serum anticardiolipin antibodies in patients with neuromyelitis optica spectrum disorder. J. Neurol. 260, 3150–3157 (2013).
Sacharidou, A., Shaul, P. W. & Mineo, C. New insights in the pathophysiology of antiphospholipid syndrome. Semin. Thromb. Hemost. 44, 475–482 (2018).
Oshiro, B. T., Silver, R. M., Scott, J. R., Yu, H. & Branch, D. W. Antiphospholipid antibodies and fetal death. Obstet. Gynecol. 87, 489–493 (1996).
Gilhus, N. E. Myasthenia gravis. N. Engl. J. Med. 375, 2570–2581 (2016).
Jarius, S. et al. Neuromyelitis optica spectrum disorders in patients with myasthenia gravis: ten new aquaporin-4 antibody positive cases and a review of the literature. Mult. Scler. 18, 1135–1143 (2012).
Leite, M. I. et al. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology 78, 1601–1607 (2012).
McKeon, A. et al. Coexistence of myasthenia gravis and serological markers of neurological autoimmunity in neuromyelitis optica. Muscle Nerve 39, 87–90 (2009).
Massey, J. M. & De Jesus-Acosta, C. Pregnancy and myasthenia gravis. Continuum 20, 115–127 (2014).
Chaudhry, S. A., Vignarajah, B. & Koren, G. Myasthenia gravis during pregnancy. Can. Fam. Physician 58, 1346–1349 (2012).
Zifman, E., Litmanovitz, I., Segal, G., Regev, R. & Watemberg, N. Marked hypotonia in an infant of a mother with Devic disease. J. Child. Neurol. 25, 746–747 (2010).
Sherman, E. & Han, M. H. Acute and chronic management of neuromyelitis optica spectrum disorder. Curr. Treat. Options Neurol. 17, 48 (2015).
Das, G. et al. Rituximab before and during pregnancy: a systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 5, e453 (2018). This paper suggests that rituximab treatment before or during pregnancy does not increase the risk of birth defects, but might cause B cell depletion, depending on when fetal exposure occurred.
Vukusic, S. et al. Pregnancy outcomes in patients with multiple sclerosis and other autoimmune diseases treated with ocrelizumab in clinical trials and post-marketing studies [abstract P600]. Mult. Scler. 24 (Suppl. 2), 293 (2018).
Pfizer. Solu-Medrone 40mg. emc https://www.medicines.org.uk/emc/product/1550/smpc (2019).
Zentiva. Prednisolone 25mg tablets. emc https://www.medicines.org.uk/emc/product/4204/smpc (2019).
Blanford, A. T. & Pearson Murphy, B. E. In vitro metabolism of prednisolone, dexamethasone, betamethasone, and cortisol by the human placenta. Am. J. Obstet. Gynecol. 127, 264–267 (1977).
Kemp, M. W., Newnham, J. P., Challis, J. G., Jobe, A. H. & Stock, S. J. The clinical use of corticosteroids in pregnancy. Hum. Reprod. Update 22, 240–259 (2016).
European Medicines Agency. Neofordex, INN-dexamethasone. Annex I: summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/neofordex-epar-product-information_en.pdf (2019).
Walker, B. E. Induction of cleft palate in rabbits by several glucocorticoids. Proc. Soc. Exp. Biol. Med. 125, 1281–1284 (1967).
Pinsky, L. & DiGeorge, A. M. Cleft palate in the mouse: a teratogenic index of glucocorticoid potency. Science 147, 402–403 (1965).
Shah, R. M. & Chaudhry, A. P. Hydrocortisone-induced cleft palate in hamsters. Teratology 7, 191–194 (1973).
Pradat, P. et al. First trimester exposure to corticosteroids and oral clefts. Birth Defects Res. A Clin. Mol. Teratol. 67, 968–970 (2003).
Kallen, B. & Olausson, P. O. No increased risk of infant hypospadias after maternal use of loratadine in early pregnancy. Int. J. Med. Sci. 3, 106–107 (2006).
Hviid, A. & Mølgaard-Nielsen, D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ 183, 796–804 (2011).
Skuladottir, H. et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Res. A Clin. Mol. Teratol. 100, 499–506 (2014).
Bandoli, G., Palmsten, K., Forbess Smith, C. J. & Chambers, C. D. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum. Dis. Clin. North Am. 43, 489–502 (2017).
Tegethoff, M., Greene, N., Olsen, J., Schaffner, E. & Meinlschmidt, G. Inhaled glucocorticoids during pregnancy and offspring pediatric diseases: a national cohort study. Am. J. Respir. Crit. Care Med. 185, 557–563 (2012).
Ali Khan, A. et al. Does in utero exposure to synthetic glucocorticoids influence birthweight, head circumference and birth length? A systematic review of current evidence in humans. Paediatric Perinat. Epidemiol. 25, 20–36 (2011).
Khalife, N. et al. Prenatal glucocorticoid treatment and later mental health in children and adolescents. PLOS ONE 8, e81394 (2013).
Hirvikoski, T. et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J. Clin. Endocrinol. Metab. 92, 542–548 (2007).
Trautman, P. D., Meyer-Bahlburg, H. F. L., Postelnek, J. & New, M. I. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology 20, 439–449 (1995).
Laugesen, K., Byrjalsen, A., Frøslev, T., Olsen, M. S. & Sørensen, H. T. Use of glucocorticoids during pregnancy and risk of attention-deficit/hyperactivity disorder in offspring: a nationwide Danish cohort study. BMJ Open 7, e016825 (2017).
Liu, J., Feng, Z. C., Li, J. & Wang, Q. Antenatal dexamethasone has no adverse effects on child physical and cognitive development: a long-term cohort follow-up investigation. J. Matern. Fetal Neonatal Med. 25, 2369–2371 (2012).
de Steenwinkel, F. D. O., Dolhain, R. J. E. M., Hazes, J. M. W. & Hokken-Koelega, A. C. S. Does prednisone use or disease activity in pregnant women with rheumatoid arthritis influence the body composition of their offspring? Reprod. Toxicol. 71, 118–123 (2017).
Katz, F. H. & Duncan, B. R. Letter: entry of prednisone into human milk. N. Engl. J. Med. 293, 1154 (1975).
Öst, L., Wettrell, G., Björkhem, I. & Rane, A. Prednisolone excretion in human milk. J. Pediatr. 106, 1008–1011 (1985).
Boz, C. et al. Safety of IV pulse methylprednisolone therapy during breastfeeding in patients with multiple sclerosis. Mult. Scler. 24, 1205–1211 (2018).
Hale, T. W. Hale’s Medications & Mothers’ Milk Vol. 18 (Springer, 2018).
National Library of Medicine. Drugs and Lactation Database (LactMed) Prednisone. https://www.ncbi.nlm.nih.gov/books/NBK501077 (2018).
Constantinescu, S. et al. Breast-feeding after transplantation. Best. Pract. Res. Clin. Obstet. Gynaecol. 28, 1163–1173 (2014).
Ito, S., Blajchman, A., Stephenson, M., Eliopoulos, C. & Koren, G. Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am. J. Obstet. Gynecol. 168, 1393–1399 (1993).
National Library of Medicine. Drugs and Lactation Database (LactMed) Dexamethasone. https://www.ncbi.nlm.nih.gov/books/NBK501767 (2018).
Marathias, V. M., Sawicki, M. J. & Bolton, P. H. 6-Thioguanine alters the structure and stability of duplex DNA and inhibits quadruplex DNA formation. Nucleic Acids Res. 27, 2860–2867 (1999).
Nagafuchi, K. & Miyazaki, K. Modulation of genotoxicity of azathioprine by intracellular glutathione in hepatocytes. J. Cancer Res. Clin. Oncol. 117, 321–325 (1991).
Lennard, L. The clinical pharmacology of 6-mercaptopurine. Eur. J. Clin. Pharmacol. 43, 329–339 (1992).
Saarikoski, S. & Seppala, M. Immunosuppression during pregnancy: transmission of azathioprine and its metabolites from the mother to the fetus. Am. J. Obstet. Gynecol. 115, 1100–1106 (1973).
de Boer, N. K. H. et al. Azathioprine use during pregnancy: unexpected intrauterine exposure to metabolites. Am. J. Gastroenterol. 101, 1390–1392 (2006).
Williamson, R. A. & Karp, L. E. Azathioprine teratogenicity: review of the literature and case report. Obstet. Gynecol. 58, 247–250 (1981).
Polifka, J. E. & Friedman, J. M. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 65, 240–261 (2002).
Githens, J. H., Rosenkrantz, J. G. & Tunnock, S. M. Teratogenic effects of azathioprine (Imuran). J. Pediatrics 66, 959–961 (1965).
Rosenkrantz, J. G., Githens, J. H., Cox, S. M. & Kellum, D. L. Azathioprine (Imuran) and pregnancy. Am. J. Obstet. Gynecol. 97, 387–394 (1967).
Mylan. Azathioprine tablets 50mg. emc https://www.medicines.org.uk/emc/product/2541/smpc (2017).
Clark, J. M. The mutagenicity of azathioprine in mice, Drosophila melanogaster and Neurospora crassa. Mutat. Res. 28, 87–99 (1975).
Ligumsky, M., Badaan, S., Lewis, H. & Meirow, D. Effects of 6-mercaptopurine treatment on sperm production and reproductive performance: a study in male mice. Scand. J. Gastroenterol. 40, 444–449 (2005).
Nørgård, B., Fonager, K., Pedersen, L., Jacobsen, B. A. & Sørensen, H. T. Birth outcome in women exposed to 5-aminosalicylic acid during pregnancy: a Danish cohort study. Gut 52, 243–247 (2003).
Francella, A. et al. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: a retrospective cohort study. Gastroenterology 124, 9–17 (2003).
Moskovitz, D. N. et al. The effect on the fetus of medications used to treat pregnant inflammatory bowel-disease patients. Am. J. Gastroenterol. 99, 656–661 (2004).
Coelho, J. et al. Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME study. Gut 60, 198–203 (2011).
Akbari, M., Shah, S., Velayos, F. S., Mahadevan, U. & Cheifetz, A. S. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm. Bowel Dis. 19, 15–22 (2013). This meta-analysis indicates that treatment with azathioprine during pregnancy or at the time of conception does not increase the risk of birth defects.
Naqvi, R. et al. Outcome of pregnancy in renal allograft recipients: SIUT experience. Transplant. Proc. 38, 2001–2002 (2006).
Cleary, B. J. & Kallen, B. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defects Res. A Clin. Mol. Teratol. 85, 647–654 (2009).
Goldstein, L. H. et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 79, 696–701 (2007).
Flint, J. D., Mouyis, M. & Giles, I. A systematic review of the impact of anti-rheumatic drugs upon male fertility and paternal exposure peri-conception [abstract]. Arthritis Rheumatol. 69 (Suppl. 10), 1810 (2017).
Norgard, B., Pedersen, L., Jacobsen, J., Rasmussen, S. N. & Sorensen, H. T. The risk of congenital abnormalities in children fathered by men treated with azathioprine or mercaptopurine before conception. Aliment. Pharmacol. Ther. 19, 679–685 (2004).
Dejaco, C. et al. Azathioprine treatment and male fertility in inflammatory bowel disease. Gastroenterology 121, 1048–1053 (2001).
Sau, A. et al. Azathioprine and breastfeeding — is it safe? BJOG 114, 498–501 (2007).
Christensen, L. A., Dahlerup, J. F., Nielsen, M. J., Fallingborg, J. F. & Schmiegelow, K. Azathioprine treatment during lactation. Aliment. Pharmacol. Ther. 28, 1209–1213 (2008).
Natekar, A., Pupco, A., Bozzo, P. & Koren, G. Safety of azathioprine use during pregnancy. Can. Fam. Physician 57, 1401–1402 (2011).
Alami, Z. et al. Pregnancy outcome following in utero exposure to azathioprine: a French comparative observational study. Therapie 73, 199–207 (2018).
Mozaffari, S., Abdolghaffari, A. H., Nikfar, S. & Abdollahi, M. Pregnancy outcomes in women with inflammatory bowel disease following exposure to thiopurines and antitumor necrosis factor drugs: a systematic review with meta-analysis. Hum. Exp. Toxicol. 34, 445–459 (2015).
Bullingham, R. E. S., Nicholls, A. J. & Kamm, B. R. Clinical pharmacokinetics of mycophenolate mofetil. Clin. Pharmacokinet. 34, 429–455 (1998).
European Medicines Agency. CellCept, mycophenolate mofetil. Annex I: summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/cellcept-epar-product-information_en.pdf
Klieger-Grossmann, C. et al. Prenatal exposure to mycophenolate mofetil: an updated estimate. J. Obstet. Gynaecol. Can. 32, 794–797 (2010).
Anderka, M. T., Lin, A. E., Abuelo, D. N., Mitchell, A. A. & Rasmussen, S. A. Reviewing the evidence for mycophenolate mofetil as a new teratogen: case report and review of the literature. Am. J. Med. Genet. A 149A, 1241–1248 (2009).
Perez-Aytes, A. et al. Mycophenolate mofetil embryopathy: a newly recognized teratogenic syndrome. Eur. J. Med. Genet. 60, 16–21 (2017).
Jones, A. et al. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog. Transplant. 23, 153–157 (2013).
Midtvedt, K., Bergan, S., Reisæter, A. V., Vikse, B. E. & Åsberg, A. Exposure to mycophenolate and fatherhood. Transplantation 101, e214–e217 (2017).
Bannwarth, B., Pehourcq, F., Schaeverbeke, T. & Dehais, J. Clinical pharmacokinetics of low-dose pulse methotrexate in rheumatoid arthritis. Clin. Pharmacokinet. 30, 194–210 (1996).
Feldkamp, M. & Carey, J. C. Clinical teratology counseling and consultation case report: low dose methotrexate exposure in the early weeks of pregnancy. Teratology 47, 533–539 (1993).
Weber-Schoendorfer, C. et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 66, 1101–1110 (2014).
European Medicines Agency. Nordimet, methotrexate. Annex I: summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/nordimet-epar-product-information_en.pdf (2018).
Leroy, C. et al. Immunosuppressive drugs and fertility. Orphanet J. Rare Dis. 10, 136 (2015).
Gutierrez, J. C. & Hwang, K. The toxicity of methotrexate in male fertility and paternal teratogenicity. Expert Opin. Drug Metab. Toxicol. 13, 51–58 (2017).
French, A. E. & Koren, G. Effect of methotrexate on male fertility. Can. Fam. Physician 49, 577–578 (2003).
Eck, L. K. et al. Risk of adverse pregnancy outcome after paternal exposure to methotrexate within 90 days before pregnancy. Obstet. Gynecol. 129, 707–714 (2017).
Johns, D. G., Rutherford, L. D., Leighton, P. C. & Vogel, C. L. Secretion of methotrexate into human milk. Am. J. Obstet. Gynecol. 112, 978–980 (1972).
Thorne, J. C., Nadarajah, T., Moretti, M. & Ito, S. Methotrexate use in a breastfeeding patient with rheumatoid arthritis. J. Rheumatol. 41, 2332 (2014).
Kavanaugh, A. et al. Proceedings from the American College of Rheumatology Reproductive Health Summit: the management of fertility, pregnancy, and lactation in women with autoimmune and systemic inflammatory diseases. Arthritis Care Res. 67, 313–325 (2015).
van der Woude, C. J. et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J. Crohns Colitis 9, 107–124 (2015).
Nguyen, G. C. et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 150, 734–757.e1 (2016).
Moretti, M. E., Lee, A. & Ito, S. Which drugs are contraindicated during breastfeeding? Practice guidelines. Can. Fam. Physician 46, 1753–1757 (2000).
Pacifici, G. M. & Nottoli, R. Placental transfer of drugs administered to the mother. Clin. Pharmacokinet. 28, 235–269 (1995).
Sockolosky, J. T. & Szoka, F. C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 91, 109–124 (2015).
Palmeira, P., Quinello, C., Silveira-Lessa, A. L., Zago, C. A. & Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 985646 (2012).
Haghikia, A. et al. Natalizumab use during the third trimester of pregnancy. JAMA Neurol. 71, 891–895 (2014).
Witzel, S. J. Lactation and the use of biologic immunosuppressive medications. Breastfeed. Med. 9, 543–546 (2014).
Bragnes, Y., Boshuizen, R., de Vries, A., Lexberg, A. & Ostensen, M. Low level of rituximab in human breast milk in a patient treated during lactation. Rheumatology 56, 1047–1048 (2017).
Vesga, L., Terdiman, J. P. & Mahadevan, U. Adalimumab use in pregnancy. Gut 54, 890 (2005).
Saito, J. et al. Tocilizumab concentrations in maternal serum and breast milk during breastfeeding and a safety assessment in infants: a case study. Rheumatology 57, 1499–1501 (2018).
Kelly, R. J. et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 373, 1032–1039 (2015).
Yarur, A. & Kane, S. V. Update on pregnancy and breastfeeding in the era of biologics. Dig. Liver Dis. 45, 787–794 (2013).
Vaidyanathan, A. et al. Developmental immunotoxicology assessment of rituximab in cynomolgus monkeys. Toxicol. Sci. 119, 116–125 (2011).
Chakravarty, E. F., Murray, E. R., Kelman, A. & Farmer, P. Pregnancy outcomes after maternal exposure to rituximab. Blood 117, 1499–1506 (2011).
Garcia-Enguidanos, A., Calle, M. E., Valero, J., Luna, S. & Dominguez-Rojas, V. Risk factors in miscarriage: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 102, 111–119 (2002).
Muglia, L. J. & Katz, M. The enigma of spontaneous preterm birth. N. Engl. J. Med. 362, 529–535 (2010).
Grunewald, S. & Jank, A. New systemic agents in dermatology with respect to fertility, pregnancy, and lactation. J. Dtsch. Dermatol. Ges. 13, 277–290 (2015).
Miranda-Acuna, J. et al. Rituximab during pregnancy in neuromyelitis optica: a case report. Neurol. Neuroimmunol. Neuroinflamm. 6, e542 (2019).
Thurlings, R. M. et al. Clinical response, pharmacokinetics, development of human anti-chimaeric antibodies, and synovial tissue response to rituximab treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 69, 409–412 (2010).
European Medicines Agency. MabThera, INN-rituximab. Annex I: summary of product characteristics. EMA http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdf (2009).
Thone, J., Thiel, S., Gold, R. & Hellwig, K. Treatment of multiple sclerosis during pregnancy — safety considerations. Expert Opin. Drug Saf. 16, 523–534 (2017).
European Medicines Agency. Ocrevus, INN-ocrelizumab. Annex I: summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf (2018).
Nishimoto, N. et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J. Rheumatol. 30, 1426–1435 (2003).
European Medicines Agency. RoActemra. INN-tocilizumab. Annex I: summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_en.pdf (2019).
Weber-Schoendorfer, C. & Schaefer, C. Pregnancy outcome after tocilizumab therapy in early pregnancy-a case series from the German Embryotox Pharmacovigilance Center. Reprod. Toxicol. 60, 29–32 (2016).
Nakajima, K. et al. Pregnancy outcomes after exposure to tocilizumab: a retrospective analysis of 61 patients in Japan. Mod. Rheumatol. 26, 667–671 (2016).
Hoeltzenbein, M. et al. Tocilizumab use in pregnancy: analysis of a global safety database including data from clinical trials and post-marketing data. Semin. Arthritis Rheum. 46, 238–245 (2016). This analysis identified a slightly higher risk of miscarriage and preterm birth in women who received tocilizumab shortly before or during pregnancy than in the general population.
Borrell, A. & Stergiotou, I. Miscarriage in contemporary maternal-fetal medicine: targeting clinical dilemmas. Ultrasound Obstet. Gynecol. 42, 491–497 (2013).
Wacker, E. et al. Does the average drug exposure in pregnant women affect pregnancy outcome? A comparison of two approaches to estimate the baseline risks of adverse pregnancy outcome. Pharmacoepidemiol. Drug Saf. 24, 353–360 (2015).
Dolk, H., Loane, M. & Garne, E. The prevalence of congenital anomalies in Europe. Adv. Exp. Med. Biol. 686, 349–364 (2010).
Scheuerle, A., Vannappagari, V. X. & Miller, M. K. Measurements of birth defect prevalence: which is most useful as a comparator group for pharmaceutical pregnancy registries? Birth Defects Res. A Clin. Mol. Teratol. 85, 611–620 (2009).
Carey, J. C., Martinez, L., Balken, E., Leen-Mitchell, M. & Robertson, J. Determination of human teratogenicity by the astute clinician method: review of illustrative agents and a proposal of guidelines. Birth Defects Res. A Clin. Mol. Teratol. 85, 63–68 (2009).
Wallenius, M., Salvesen, K. A., Daltveit, A. K. & Skomsvoll, J. F. Miscarriage and stillbirth in women with rheumatoid arthritis. J. Rheumatol. 42, 1570–1572 (2015).
Bharti, B. et al. Disease severity and pregnancy outcomes in women with rheumatoid arthritis: results from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project. J. Rheumatol. 42, 1376–1382 (2015).
Langen, E. S., Chakravarty, E. F., Liaquat, M., El-Sayed, Y. Y. & Druzin, M. L. High rate of preterm birth in pregnancies complicated by rheumatoid arthritis. Am. J. Perinatol. 31, 9–14 (2014).
Ehninger, G., Schuler, U., Proksch, B., Zeller, K. P. & Blanz, J. Pharmacokinetics and metabolism of mitoxantrone. A review. Clin. Pharmacokinet. 18, 365–380 (1990).
European Medicines Agency. Novantrone EMEA-H-A-30-1399. Annex III: summary of product characteristics, labelling and package leaflet. EMA http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Novantrone_30/WC500205489.pdf (2016)
Giacalone, P. L., Laffargue, F. & Benos, P. Chemotherapy for breast carcinoma during pregnancy: a French national survey. Cancer 86, 2266–2272 (1999).
Hellwig, K., Schimrigk, S., Chan, A., Epplen, J. & Gold, R. A newborn with Pierre Robin sequence after preconceptional mitoxantrone exposure of a female with multiple sclerosis. J. Neurol. Sci. 307, 164–165 (2011).
Azuno, Y. et al. Mitoxantrone and etoposide in breast milk. Am. J. Hematol. 48, 131–132 (1995).
Meistrich, M. L. et al. Rapid recovery of spermatogenesis after mitoxantrone, vincristine, vinblastine, and prednisone chemotherapy for Hodgkin’s disease. J. Clin. Oncol. 15, 3488–3495 (1997).
Cocco, E. et al. Frequency and risk factors of mitoxantrone-induced amenorrhea in multiple sclerosis: the FEMIMS study. Mult. Scler. 14, 1225–1233 (2008).
Bontadi, A. et al. Plasma exchange and immunoadsorption effectively remove antiphospholipid antibodies in pregnant patients with antiphospholipid syndrome. J. Clin. Apher. 27, 200–204 (2012).
El-Haieg, D. O., Zanati, M. F. & El-Foual, F. M. Plasmapheresis and pregnancy outcome in patients with antiphospholipid syndrome. Int. J. Gynecol. Obstet. 99, 236–241 (2007).
Abou-Nassar, K., Karsh, J., Giulivi, A. & Allan, D. Successful prevention of thrombotic thrombocytopenic purpura (TTP) relapse using monthly prophylactic plasma exchanges throughout pregnancy in a patient with systemic lupus erythematosus and a prior history of refractory TTP and recurrent fetal loss. Transfus. Apher. Sci. 43, 29–31 (2010).
Proia, A. et al. Thrombotic thrombocytopenic purpura and pregnancy: a case report and a review of the literature. Ann. Hematol. 81, 210–214 (2002).
Levine, S. E. & Keesey, J. C. Successful plasmapheresis for fulminant myasthenia gravis during pregnancy. Arch. Neurol. 43, 197–198 (1986).
Batocchi, A. P. et al. Course and treatment of myasthenia gravis during pregnancy. Neurology 52, 447–447 (1999).
Djelmis, J., Sostarko, M., Mayer, D. & Ivanisevic, M. Myasthenia gravis in pregnancy: report on 69 cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 104, 21–25 (2002).
Trebst, C. et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J. Neurol. 261, 1–16 (2014).
Kleiter, I. et al. Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions. Neurol. Neuroimmunol. Neuroinflamm. 5, e504 (2018).
Watson, W. J., Katz, V. L. & Bowes, W. A. Jr. Plasmapheresis during pregnancy. Obstet. Gynecol. 76, 451–457 (1990).
Cox, J. L., Koepsell, S. A. & Shunkwiler, S. M. Therapeutic plasma exchange and pregnancy: a case report and guidelines for performing plasma exchange in a pregnant patient. J. Clin. Apher. 32, 191–195 (2017).
Hoffmann, F. et al. Tryptophan immunoadsorption during pregnancy and breastfeeding in patients with acute relapse of multiple sclerosis and neuromyelitis optica. Ther. Adv. Neurol. Disord. 11, 1756286418774973 (2018).
Nakamura, Y. et al. Immunoadsorption plasmapheresis as a treatment for pregnancy complicated by systemic lupus erythematosus with positive antiphospholipid antibodies. Am. J. Reprod. Immunol. 41, 307–311 (1999).
Wang, C., Wolf, S., Khan, M. & Mao-Draayer, Y. Interleukin-6 receptor: a novel therapeutic target for neuromyelitis optica. Brain Disord. Ther. 4, e119 (2015).
Yamamura, T. et al. Efficacy of satralizumab (SA237) in subgroups of patients in SAkuraSky: a phase III double-blind, placebo-controlled, add-on study in patients with neuromyelitis optica spectrum disorder (NMOSD) [abstract WCN19-2024]. J. Neurol. Sci. 405, 11–12 (2019).
Gatault, P. et al. Therapeutic drug monitoring of eculizumab: rationale for an individualized dosing schedule. MAbs 7, 1205–1211 (2015).
European Medicines Agency. Soliris, INN-eculizumab. Annex I: summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/soliris-epar-product-information_en.pdf (2019).
Alexion. Alexion announces successful phase 3 PREVENT study of Soliris® (eculizumab) in patients with neuromyelitis optica spectrum disorder (NMOSD). Alexion Pharma https://news.alexionpharma.com/press-release/product-news/alexion-announces-successful-phase-3-prevent-study-soliris-eculizumab-pat (2018).
Sarno, L. et al. Eculizumab in pregnancy: a narrative overview. J. Nephrol. 32, 17–25 (2019).
Hallstensen, R. F. et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 220, 452–459 (2015).
Kelly, R. et al. The management of pregnancy in paroxysmal nocturnal haemoglobinuria on long term eculizumab. Br. J. Haematol. 149, 446–450 (2010).
Chen, D., Gallagher, S., Monson, N. L., Herbst, R. & Wang, Y. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies. J. Clin. Med. 5, 107 (2016).
Agius, M. A. et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult. Scler. 25, 235–245 (2019).
Cree, B. A. C. et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 394, 1352–1363 (2019).
European Medicines Agency. Cellcept, INN-mycophenolate mofetil. Annex I: summary of product characteristics. EMA https://www.ema.europa.eu/en/documents/product-information/cellcept-epar-product-information_en.pdf (2018).
Acknowledgements
K.H. is an investigator of the NEMOS cohort/NationNMO supported by the German Ministry for Education and Research (BMBF) as part of the ‘German Competence Network Multiple Sclerosis’ (KKNMS; FKZ 01GI1602). She is also a member of the international Guthy-Jackson Charitable Foundation (GJCF) and coordinator of the GJCF International Clinical Consortium Sex & Gender Specificity Working Group, and receives grant support from the Innovation Fund of the Federal Joint Committee. I.K.S. has received research funding from the National Multiple Sclerosis Society, the US Department of Defense and the Guthy-Jackson Charitable Foundation. S.J. thanks the Dietmar Hopp Foundation and Merck Serono for funding research on AQP4-IgG-positive NMOSD and on MOG encephalomyelitis. Y.M.-D. is currently supported by grants from the NIH National Institute of Allergy and Infectious Diseases Autoimmune Center of Excellence (UM1-AI110557; NIH NINDS R01-NS080821). E.M. was supported by a Kirschstein-NRSA (2T32HD007505–21) grant. T.C. is currently supported by grants from the Department of Defense, the Guthy-Jackson Charitable Foundation and the National MS Society. S.T. is currently supported by the Innovation Fund of the Federal Joint Committee. M.I.L. receives support from the National Health Service National Specialized Commissioning Group for Neuromyelitis Optica UK and the National Institute for Health Research Oxford Biomedical Research Centre. The authors thank C. Fisher for her assistance in designing the figures for this Review.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
Y.M.-D. has served as a consultant, speaker and/or received grant support from Acorda, Bayer Pharmaceutical, Biogen Idec, Celgene, Chugai, EMD Serono, Novartis, Questor, Roche-Genentech, Sanofi-Genzyme and Teva Neuroscience. T.C. has served as a one-time consultant for Alexion. M.F. has received speaker honoraria from Biogen Idec. M.I.L. received travel funding and speaker honoraria from Biogen Idec and received a travel grant from Novartis. The work of S.J. was indirectly supported by grants from Dietmar Hopp Stiftung, Germany, and from Merck Serono, Germany (to B. Wildemann, Department of Neurology, University Hospital Heidelberg). K.H. received research support and speaker honoraria from Bayer, Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva. E.M., I.K.S. and S.T. declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks J. de Seze, H. J. Kim and M. Levy for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Mycophenolate Risk Evaluation and Mitigation Strategy: https://www.mycophenolaterems.com/
Rights and permissions
About this article
Cite this article
Mao-Draayer, Y., Thiel, S., Mills, E.A. et al. Neuromyelitis optica spectrum disorders and pregnancy: therapeutic considerations. Nat Rev Neurol 16, 154–170 (2020). https://doi.org/10.1038/s41582-020-0313-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-0313-y
This article is cited by
-
Update on the diagnosis and treatment of neuromyelitis optica spectrum disorders (NMOSD) – revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part II: Attack therapy and long-term management
Journal of Neurology (2024)
-
Contemporary management challenges in seropositive NMOSD
Journal of Neurology (2022)
-
Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies
Journal of Neuroinflammation (2021)
-
New Therapeutic Landscape in Neuromyelitis Optica
Current Treatment Options in Neurology (2021)
-
Recent progress in maintenance treatment of neuromyelitis optica spectrum disorder
Journal of Neurology (2021)