Abstract
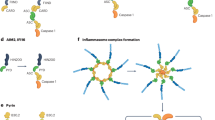
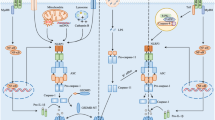
The mammalian CNS is an intricate and fragile structure, which on one hand is open to change in order to store information, but on the other hand is vulnerable to damage from injury, pathogen invasion or neurodegeneration. During senescence and neurodegeneration, activation of the innate immune system can occur. Inflammasomes are signalling complexes that regulate cells of the immune system, which in the brain mainly includes microglial cells. In microglia, the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome becomes activated when these cells sense proteins such as misfolded or aggregated amyloid-β, α-synuclein and prion protein or superoxide dismutase, ATP and members of the complement pathway. Several other inflammasomes have been described in microglia and the other cells of the brain, including astrocytes and neurons, where their activation and subsequent caspase 1 cleavage contribute to disease development and progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
11 February 2019
In the originally published version of this article, the competing interests statement indicated that the authors had no competing interests; however, this statement was incorrect. The statement should have read as follows: ‘M.H. receives a consultation fee from IFM Therapeutics, LLC for consultations regarding the pathogenesis and interventional strategies of neurodegenerative disease. E.L. is a scientific co-founder and consultant to IFM Therapeutics. R.M.M. declares no competing interests.’ This error has been corrected in the HTML and PDF versions of the article.
References
Cooper, M. D. & Alder, M. N. The evolution of adaptive immune systems. Cell 124, 815–822 (2006).
Janeway, C. A. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Matzinger, P. An innate sense of danger. Semin. Immunol. 10, 399–415 (1998).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32, 461–488 (2014).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Mehta, M. M., Weinberg, S. E. & Chandel, N. S. Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol. 17, 608–620 (2017).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443 (2015).
Zhao, Y. & Shao, F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol. Rev. 265, 85–102 (2015).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513, 237–241 (2014).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Próchnicki, T. & Latz, E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 26, 71–93 (2017).
Maturana, C. J., Aguirre, A. & Sáez, J. C. High glucocorticoid levels during gestation activate the inflammasome in hippocampal oligodendrocytes of the offspring. Dev. Neurobiol. 77, 625–642 (2017).
Johann, S. et al. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63, 2260–2273 (2015).
Liu, H.-D. et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem. Res. 38, 2072–2083 (2013).
Tan, M.-S. et al. Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 5, e1382 (2014).This paper shows that Aβ can activate the NLRP1 inflammasome in neurons, leading to pyroptosis and cognitive impairments.
Silverman, W. R. et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 284, 18143–18151 (2009).
Kaushal, V. et al. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 22, 1676–1686 (2015).
Burm, S. M. et al. Inflammasome-induced IL-1β secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J. Neurosci. 35, 678–687 (2015).
Walsh, J. G. et al. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology 11, 35 (2014).
Nyúl-Tóth, Á. et al. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain Behav. Immun. 64, 220–231 (2017).
Minkiewicz, J., de Rivero Vaccari, J. P. & Keane, R. W. Human astrocytes express a novel NLRP2 inflammasome. Glia 61, 1113–1121 (2013).
Liu, L. & Chan, C. IPAF inflammasome is involved in interleukin-1β production from astrocytes, induced by palmitate; implications for Alzheimer’s disease. Neurobiol. Aging 35, 309–321 (2014).
Adamczak, S. E. et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J. Cereb. Blood Flow Metab. 34, 621–629 (2014).
Nagyőszi, P. et al. Regulation of NOD-like receptors and inflammasome activation in cerebral endothelial cells. J. Neurochem. 135, 551–564 (2015).
Burm, S. M., Zuiderwijk-Sick, E. A., Weert, P. M. & Bajramovic, J. J. ATP-induced IL-1β secretion is selectively impaired in microglia as compared to hematopoietic macrophages. Glia 64, 2231–2246 (2016).
Freeman, L. et al. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 214, 1351–1370 (2017).This paper shows that astrocytes also have functional NLRP3 and NLRC4 inflammasomes, and, interestingly, NLRC4 was detected in the brains of patients with multiple sclerosis.
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857–865 (2008).This study first demonstrated that Aβ activates the NLRP3 inflammasome.
Deora, V., Albornoz, E. A., Zhu, K., Woodruff, T. M. & Gordon, R. The ketone body β-hydroxybutyrate does not inhibit synuclein mediated inflammasome activation in microglia. J. Neuroimmune Pharmacol. 12, 568–574 (2017).
Codolo, G. et al. Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 8, e55375 (2013).
Zhou, Y. et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 11, 28 (2016).
Zhao, W. et al. TDP-43 activates microglia through NF-κB and NLRP3 inflammasome. Exp. Neurol. 273, 24–35 (2015).
Won, J.-H., Park, S., Hong, S., Son, S. & Yu, J.-W. Rotenone-induced impairment of mitochondrial electron transport chain confers a selective priming signal for NLRP3 inflammasome activation. J. Biol. Chem. 290, 27425–27437 (2015).
Zielinski, M. R. et al. The NLRP3 inflammasome modulates sleep and NREM sleep delta power induced by spontaneous wakefulness, sleep deprivation and lipopolysaccharide. Brain Behav. Immun. 62, 137–150 (2017).
Ju, Y.-E. S., Lucey, B. P. & Holtzman, D. M. Sleep and Alzheimer disease pathology — a bidirectional relationship. Nat. Rev. Neurol. 10, 115–119 (2014).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Tan, F. C. C., Hutchison, E. R., Eitan, E. & Mattson, M. P. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology 15, 643–660 (2014).
Akiyama, H. et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421 (2000).
Tha, K. K. et al. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 885, 25–31 (2000).
Youm, Y.-H. et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 (2013).This study shows that NLRP3 has an important role in age-related inflammation in both the brain and the periphery.
Norden, D. M. & Godbout, J. P. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39, 19–34 (2013).
Wu, Z. et al. Differential pathways for interleukin-1β production activated by chromogranin A and amyloid β in microglia. Neurobiol. Aging 34, 2715–2725 (2013).
Henry, C. J. et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflamm. 5, 15 (2008).
Richwine, A. F. et al. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology 33, 1369–1377 (2008).
Xie, Z., Morgan, T. E., Rozovsky, I. & Finch, C. E. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp. Neurol. 182, 135–141 (2003).
Huang, Y., Henry, C. J., Dantzer, R., Johnson, R. W. & Godbout, J. P. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol. Aging 29, 1744–1753 (2008).
Shaftel, S. S., Griffin, W. S. T. & O’Banion, M. K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflamm. 5, 7 (2008).
Sims, R. et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384 (2017).This study demonstrates that microglial-mediated innate immunity is implicated in AD.
Griciuc, A. et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78, 631–643 (2013).
Morales, I., Jiménez, J. M., Mancilla, M. & Maccioni, R. B. Tau oligomers and fibrils induce activation of microglial cells. J. Alzheimers Dis. 37, 849–856 (2013).
Sanchez-Mejias, E. et al. Soluble phospho-tau from Alzheimer’s disease hippocampus drives microglial degeneration. Acta Neuropathol. 132, 897–916 (2016).
Heneka, M. T., Golenbock, D. T. & Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 16, 229–236 (2015).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).This study demonstrates that inflammasome activation occurs in the brains of patients with AD, and murine studies confirm its role in pathology, as inhibition of the NLRP3 inflammasome protects transgenic mice from neuroinflammation and cognitive deficits.
Saresella, M. et al. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener. 11, 23 (2016).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating the intracellular nucleation from soluble to particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Parajuli, B. et al. Oligomeric amyloid β induces IL-1β processing via production of ROS: implication in Alzheimer’s disease. Cell Death Dis. 4, e975 (2013).
Cho, M.-H. et al. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10, 1761–1775 (2014).
Koffie, R. M. et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl Acad. Sci. USA 106, 4012–4017 (2009).
Spires, T. L. et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25, 7278–7287 (2005).
Couturier, J. et al. Activation of phagocytic activity in astrocytes by reduced expression of the inflammasome component ASC and its implication in a mouse model of Alzheimer disease. J. Neuroinflamm. 13, 20 (2016).
Franklin, B. S. et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 15, 727–737 (2014).This is the first study to demonstrate that ASC specks are released from inflammasome-activated cells, where they can further perpetuate inflammatory responses.
Leissring, M. A. et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093 (2003).
Murray, C. A. & Lynch, M. A. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J. Neurosci. 18, 2974–2981 (1998).
Venegas, C. et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017).This study shows that microglia release ASC specks that rapidly bind Aβ, increasing the formation of oligomers and aggregates, thus acting as a seed for Aβ pathology.
Fu, A. K. Y. et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl Acad. Sci. USA 113, E2705–E2713 (2016).
Chen, L., Na, R., Boldt, E. & Ran, Q. NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol. Aging 36, 2533–2543 (2015).
Dempsey, C. et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav. Immun. 61, 306–316 (2017).
Yin, J. et al. NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 55, 1977–1987 (2017).
Daniels, M. J. D. et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 7, 12504 (2016).
Wu, P.-J., Hung, Y.-F., Liu, H.-Y. & Hsueh, Y.-P. Deletion of the inflammasome sensor Aim2 mitigates Aβ deposition and microglial activation but increases inflammatory cytokine expression in an alzheimer disease mouse model. Neuroimmunomodulation 24, 29–39 (2017).
Tancredi, V. et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J. Neurochem. 75, 634–643 (2000).
Gustot, A. et al. Amyloid fibrils are the molecular trigger of inflammation in Parkinson’s disease. Biochem. J. 471, 323–333 (2015).
Daniele, S. G. et al. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal. 8, ra45 (2015).
Wang, W. et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein α-synuclein. Proc. Natl Acad. Sci. USA 113, 9587–9592 (2016). These authors show that caspase 1 localizes with α-Syn Lewy bodies in patients with PD, and, importantly, caspase 1 directly cleaves α-Syn, resulting in fragments that can quickly aggregate and are toxic to neuronal cells.
Hung, K.-C., Huang, H.-J., Wang, Y.-T. & Lin, A. M.-Y. Baicalein attenuates α-synuclein aggregation, inflammasome activation and autophagy in the MPP(+)-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 194, 522–529 (2016).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Meissner, F., Molawi, K. & Zychlinsky, A. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc. Natl Acad. Sci. USA 107, 13046–13050 (2010). This study shows that mutant SOD can activate caspase 1 cleavage in microglia in an ASC-dependent manner, thus implicating the inflammasome in the progression of ALS.
Bellezza, I. et al. Peroxynitrite activates the NLRP3 inflammasome cascade in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol. Neurobiol. 55, 2350–2361 (2017).
Italiani, P. et al. Evaluating the levels of interleukin-1 family cytokines in sporadic amyotrophic lateral sclerosis. J. Neuroinflamm. 11, 94 (2014).
Chen-Plotkin, A. S. et al. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol. 119, 111–122 (2010).
McKee, A. C., Stein, T. D., Kiernan, P. T. & Alvarez, V. E. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 25, 350–364 (2015).
Zhuang, J. et al. TDP-43 upregulation mediated by the NLRP3 inflammasome induces cognitive impairment in 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47)-treated mice. Brain Behav. Immun. 65, 99–110 (2017).
de Rivero Vaccari, J. P. et al. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 1251–1261 (2009). This paper shows that TBI induces inflammasome activation in rats with cleavage of caspase 1 and IL-1β to their mature forms; however, this is attenuated by treatment with anti-ASC neutralizing antibodies, as is the contusion size induced by injury.
Brickler, T. et al. Nonessential role for the NLRP1 inflammasome complex in a murine model of traumatic brain injury. Mediators Inflamm. 2016, 6373506 (2016).
Saylor, D. et al. HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat. Rev. Neurol. 12, 234–248 (2016).
Chivero, E. T. et al. HIV-1 tat primes and activates microglial NLRP3 inflammasome-mediated neuroinflammation. J. Neurosci. 37, 3599–3609 (2017).
Mamik, M. K. et al. HIV-1 viral protein r activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J. Neuroimmune Pharmacol. 12, 233–248 (2017).
Tricarico, P. M., Caracciolo, I., Crovella, S. & D’Agaro, P. Zika virus induces inflammasome activation in the glial cell line U87-MG. Biochem. Biophys. Res. Commun. 492, 597–602 (2017).
Kemp, S., Huffnagel, I. C., Linthorst, G. E., Wanders, R. J. & Engelen, M. Adrenoleukodystrophy — neuroendocrine pathogenesis and redefinition of natural history. Nat. Rev. Endocrinol. 12, 606–615 (2016).
Jang, J. et al. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat. Commun. 7, 13129 (2016).
Kaushik, D. K., Gupta, M., Kumawat, K. L. & Basu, A. NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLOS ONE 7, e32270 (2012).
Tan, C.-C. et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J. Neuroinflammation 12, 18 (2015).
Meng, X.-F. et al. Inhibition of the NLRP3 inflammasome provides neuroprotection in rats following amygdala kindling-induced status epilepticus. J. Neuroinflammation 11, 212 (2014).
Johnson, K. E., Chikoti, L. & Chandran, B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J. Virol. 87, 5005–5018 (2013).
Fann, D. Y.-W. et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 4, e790 (2013).
Yang, F. et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J. Cereb. Blood Flow Metab. 34, 660–667 (2014).
Kumar, M. et al. Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in west Nile virus encephalitis. J. Virol. 87, 3655–3667 (2013).
Ramos, H. J. et al. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLOS Pathog. 8, e1003039 (2012).
Acknowledgements
M.T.H. is supported by grants from the Deutsche Forschungsgesellschaft (DFG; DFG SFBs 1089, HE). E.L. is supported by grants from the DFG (DFG SFBs 645, 670 and 1123; TRRs 83 and 57), a grant from the US National Institutes of Health (1R01HL112661) and by a European Research Council Consolidator grant (InflammAct). E.L. and M.T.H. are members of the excellence cluster ImmunoSensation funded by the DFG. M.T.H. is supported by the European Union Joint Programme–Neurodegenerative Disease (JPND) consortium InCure (funding code 01ED1505A).
Reviewer information
Nature Reviews Neuroscience thanks A. LeBlanc, J. Bajramovic and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
M.T.H., R.M.M. and E.L. researched data for the article, made substantial contribution to discussion of content and contributed to the writing, review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.H. receives a consultation fee from IFM Therapeutics, LLC for consultations regarding the pathogenesis and interventional strategies of neurodegenerative disease. E.L. is a scientific co-founder and consultant to IFM Therapeutics. R.M.M. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Innate immune system
-
Evolutionarily conserved arm of the immune system that recognizes pathogens and molecules arising in danger situations via germline-encoded signalling receptors and provides the first line of defence.
- Adaptive immune system
-
The vertebrates’ immune subsystem that relies on clonal expansion of specialized immune cells in which highly specific receptors towards antigens are created through genetic recombination of antigen receptor gene segments to provide long-lasting acquired immunity.
- T lymphocytes
-
A type of lymphocyte with cytotoxic, helper, regulatory and memory functions characterized by expression of the T cell receptor.
- B lymphocytes
-
A type of lymphocyte that expresses the B cell receptor that recognizes specific antigens leading to the production of antibodies that function in providing humoral immunity.
- Cell-autonomous immunity
-
The cell intrinsic immune defence that is provided by the function of innate immune signalling receptors expressed in the individual cell.
- Inflammasome
-
Inflammasomes are multiprotein complexes, formed of an inflammasome sensor molecule with the adaptor ASC and the effector caspase 1, that mediate proteolytic activation of IL-1β family cytokines and pyroptotic cell death.
- Pyroptosis
-
An inflammatory form of programmed cell death that is triggered by inflammatory caspases after activation of inflammasomes or cytoplasmic recognition of LPS and danger-associated molecules.
- Sterile tissue inflammation
-
Inflammation induced by a variety of insults such as molecules released from dying cells that may be injured owing to trauma or crystal deposition or in chronic conditions.
- Pericontusional
-
The area of tissue surrounding a contusion or an injury in the brain, often caused by trauma or an impact to the head.
Rights and permissions
About this article
Cite this article
Heneka, M.T., McManus, R.M. & Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19, 610–621 (2018). https://doi.org/10.1038/s41583-018-0055-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-018-0055-7
This article is cited by
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
NLRP inflammasomes in health and disease
Molecular Biomedicine (2024)
-
The endotoxin hypothesis of Alzheimer’s disease
Molecular Neurodegeneration (2024)
-
A review on traditional Chinese medicine natural products and acupuncture intervention for Alzheimer’s disease based on the neuroinflammatory
Chinese Medicine (2024)
-
Differential contribution of THIK-1 K+ channels and P2X7 receptors to ATP-mediated neuroinflammation by human microglia
Journal of Neuroinflammation (2024)