Abstract
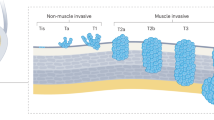
Stage T1 bladder cancers invade the lamina propria of the bladder and, despite sharing many of the genetic features of muscle-invasive bladder cancers, are classified as non-muscle-invasive or ‘superficial’ tumours. Yet, patients with T1 bladder cancer have an overall mortality of 33% and a cancer-specific mortality of 14% at three years after diagnosis, suggesting that these patients have a high risk of progression and, accordingly, require meticulous surgery, endoscopic surveillance and clinical decision-making. We hypothesize that the variability in the outcomes of patients with T1 bladder cancer is a result of both tumour heterogeneity and pathological staging, as well as inconsistencies in risk stratification, endoscopic resection and schedules of delivery of BCG. Owing to limitations in clinical staging, patients with T1 bladder cancer are at risk of both undertreatment with persistent use of BCG despite recurrence, and overtreatment with early cystectomy. Understanding the molecular features of T1 bladder cancers and how they respond to BCG therapy could improve biomarkers for risk stratification to align therapy with biological risk.
Key points
-
Non-muscle-invasive bladder cancers (NMIBCs) comprise ~70% of all bladder cancers and T1 tumours represent 20% of all NMIBCs.
-
Patients with T1 high-grade (T1HG) bladder cancer have a 10-year recurrence rate of ~77%, a 10-year progression rate of ~42% and a 10-year cancer-specific mortality of ~15%.
-
Staging T1 cancers can be challenging; substaging made on the basis of muscularis mucosae invasion has been performed, but has not been universally adopted.
-
T1 bladder cancer with aggressive features (such as lymphovascular invasion and variant histology) might warrant early cystectomy as progression to muscle-invasive disease after BCG is associated with poor survival outcomes.
-
Among patients receiving maintenance BCG therapy, data suggest that full-dose BCG therapy for 3 years is the most beneficial regimen.
-
No clear predictive biomarker of T1 bladder cancer progression has been identified; challenges remain in the identification of tumours (or patients) that are BCG unresponsive or have occult aggressive cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sjodahl, G., Eriksson, P., Liedberg, F. & Hoglund, M. Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J. Pathol. 242, 113–125 (2017).
Sjodahl, G. et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18, 3377–3386 (2012).
Choi, W. et al. Genetic alterations in the molecular subtypes of bladder cancer: illustration in the Cancer Genome Atlas Dataset. Eur. Urol. 72, 354–365 (2017).
Audenet, F., Attalla, K. & Sfakianos, J. P. The evolution of bladder cancer genomics: what have we learned and how can we use it? Urol. Oncol. 36, 313–320 (2018).
Glaser, A. P., Fantini, D., Shilatifard, A., Schaeffer, E. M. & Meeks, J. J. The evolving genomic landscape of urothelial carcinoma. Nat. Rev. Urol. 14, 215–229 (2017).
Wang, L. et al. Resetting the epigenetic balance of Polycomb and COMPASS function at enhancers for cancer therapy. Nat. Med. 24, 758–769 (2018).
Kirkali, Z. et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 66, 4–34 (2005).
Sylvester, R. J. et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 49, 466–465; discussion 475–467 (2006).
Cookson, M. S. et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J. Urol. 158, 62–67 (1997).
Gontero, P. et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: results of a retrospective multicenter study of 2451 patients. Eur. Urol. 67, 74–82 (2015).
Martin-Doyle, W., Leow, J. J., Orsola, A., Chang, S. L. & Bellmunt, J. Improving selection criteria for early cystectomy in high-grade T1 bladder cancer: a meta-analysis of 15,215 patients. J. Clin. Oncol. 33, 643–650 (2015).
Chamie, K. et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer 119, 3219–3227 (2013).
Matulewicz, R. S., Frainey, B. T., Oberlin, D. T. & Meeks, J. J. High-risk of adverse pathologic features in patients with clinical T1 high-grade bladder cancer undergoing radical cystectomy. J. Natl Compr. Canc. Netw. 14, 1403–1411 (2016).
Cambier, S. et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance Bacillus Calmette-Guerin. Eur. Urol. 69, 60–69 (2016).
van den Bosch, S. & Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur. Urol. 60, 493–500 (2011).
Schrier, B. P., Hollander, M. P., van Rhijn, B. W., Kiemeney, L. A. & Witjes, J. A. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur. Urol. 45, 292–296 (2004).
Chang, S. S. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline. J. Urol. 196, 1021–1029 (2016).
Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur. Urol. 71, 447–461 (2017).
Amin, M. B. et al. Update for the practicing pathologist: the International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer. Mod. Pathol. 28, 612–630 (2015).
Mikulowski, P. & Hellsten, S. T1 G1 urinary bladder carcinoma: fact or fiction? Scand. J. Urol. Nephrol. 39, 135–137 (2005).
Humphrey, P. A., Moch, H., Cubilla, A. L., Ulbright, T. M. & Reuter, V. E. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur. Urol. 70, 106–119 (2016).
Holmang, S. & Johansson, S. L. The nested variant of transitional cell carcinoma—a rare neoplasm with poor prognosis. Scand. J. Urol. Nephrol. 35, 102–105 (2001).
Truong, M. et al. Cautery artifact understages urothelial cancer at initial transurethral resection of large bladder tumours. Can. Urol. Assoc. J. 11, E203–E206 (2017).
van der Meijden, A., Sylvester, R., Collette, L., Bono, A. & Ten Kate, F. The role and impact of pathology review on stage and grade assessment of stages Ta and T1 bladder tumors: a combined analysis of 5 European Organization for Research and Treatment of Cancer Trials. J. Urol. 164, 1533–1537 (2000).
Bol, M. G. et al. Reproducibility and prognostic variability of grade and lamina propria invasion in stages Ta, T1 urothelial carcinoma of the bladder. J. Urol. 169, 1291–1294 (2003).
Angulo, J. C., Lopez, J. I., Grignon, D. J. & Sanchez-Chapado, M. Muscularis mucosa differentiates two populations with different prognosis in stage T1 bladder cancer. Urology 45, 47–53 (1995).
Chang, W. C., Chang, Y. H. & Pan, C. C. Prognostic significance in substaging of T1 urinary bladder urothelial carcinoma on transurethral resection. Am. J. Surg. Pathol. 36, 454–461 (2012).
Reuter, V. E. The pathology of bladder cancer. Urology 67, 11–17; discussion 17–18 (2006).
Roupret, M. et al. Prognostic interest in discriminating muscularis mucosa invasion (T1a versus T1b) in nonmuscle invasive bladder carcinoma: French national multicenter study with central pathology review. J. Urol. 189, 2069–2076 (2013).
van Rhijn, B. W. et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur. Urol. 61, 378–384 (2012).
Orsola, A. et al. Reexamining treatment of high-grade T1 bladder cancer according to depth of lamina propria invasion: a prospective trial of 200 patients. Br. J. Cancer 112, 468–474 (2015).
Dutta, S. C. et al. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J. Urol. 166, 490–493 (2001).
Dalbagni, G. Bladder cancer: restaging TUR reduces recurrence and progression risk. Nat. Rev. Urol. 7, 649–650 (2010).
Dalbagni, G., Herr, H. W. & Reuter, V. E. Impact of a second transurethral resection on the staging of T1 bladder cancer. Urology 60, 822–824; discussion 824–825 (2002).
Mariappan, P., Zachou, A. & Grigor, K. M. Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur. Urol. 57, 843–849 (2010).
Chamie, K. et al. Quality of diagnostic staging in patients with bladder cancer: a process-outcomes link. Cancer 121, 379–385 (2015).
Ark, J. T. et al. Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy. BJU Int. 113, 894–899 (2014).
Panebianco, V. et al. Improving staging in bladder cancer: the increasing role of multiparametric magnetic resonance imaging. Eur. Urol. Focus 2, 113–121 (2016).
Panebianco, V. et al. An evaluation of morphological and functional multi-parametric MRI sequences in classifying non-muscle and muscle invasive bladder cancer. Eur. Radiol. 27, 3759–3766 (2017).
Wu, L. M. et al. Clinical value of T2-weighted imaging combined with diffusion-weighted imaging in preoperative T staging of urinary bladder cancer: a large-scale, multiobserver prospective study on 3.0-T MRI. Acad. Radiol. 20, 939–946 (2013).
Algaba, F. Lymphovascular invasion as a prognostic tool for advanced bladder cancer. Curr. Opin. Urol. 16, 367–371 (2006).
Mathieu, R., Lucca, I., Roupret, M., Briganti, A. & Shariat, S. F. The prognostic role of lymphovascular invasion in urothelial carcinoma of the bladder. Nat. Rev. Urol. 13, 471–479 (2016).
Olsson, H., Hultman, P., Rosell, J. & Jahnson, S. Population-based study on prognostic factors for recurrence and progression in primary stage T1 bladder tumours. Scand. J. Urol. 47, 188–195 (2013).
Kunju, L. P. et al. Lymphovascular invasion of urothelial cancer in matched transurethral bladder tumor resection and radical cystectomy specimens. J. Urol. 180, 1928–1932; discussion 1932 (2008).
Kim, H. S. et al. Presence of lymphovascular invasion in urothelial bladder cancer specimens after transurethral resections correlates with risk of upstaging and survival: a systematic review and meta-analysis. Urol. Oncol. 32, 1191–1199 (2014).
Reuter, V. E. Lymphovascular invasion as an independent predictor of recurrence and survival in node-negative bladder cancer remains to be proven. J. Clin. Oncol. 23, 6450–6451 (2005).
Sylvester, R. J. et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology 66, 90–107 (2005).
Fernandez-Gomez, J. et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with Bacillus Calmette-Guerin: multivariate analysis of data from four randomized CUETO trials. Eur. Urol. 53, 992–1001 (2008).
Burger, M. et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur. Urol. 64, 846–854 (2013).
Wasco, M. J. et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology 70, 69–74 (2007).
Cai, T. et al. Concordance and clinical significance of uncommon variants of bladder urothelial carcinoma in transurethral resection and radical cystectomy specimens. Urology 84, 1141–1146 (2014).
Spaliviero, M. et al. Clinical outcome of patients with T1 micropapillary urothelial carcinoma of the bladder. J. Urol. 192, 702–707 (2014).
Kamat, A. M. et al. The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J. Urol. 175, 881–885 (2006).
Willis, D. L. et al. Clinical outcomes of cT1 micropapillary bladder cancer. J. Urol. 193, 1129–1134 (2015).
Seisen, T., Comperat, E., Leon, P. & Roupret, M. Impact of histological variants on the outcomes of nonmuscle invasive bladder cancer after transurethral resection. Curr. Opin. Urol. 24, 524–531 (2014).
Moschini, M. et al. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 14, 651–668 (2017).
Klaassen, Z. et al. Treatment strategy for newly diagnosed T1 high-grade bladder urothelial carcinoma: new insights and updated recommendations. Eur. Urol. https://doi.org/10.1016/j.eururo.2018.06.024 (2018).
Herr, H. W. The value of a second transurethral resection in evaluating patients with bladder tumors. J. Urol. 162, 74–76 (1999).
Herr, H. W., Donat, S. M. & Dalbagni, G. Can restaging transurethral resection of T1 bladder cancer select patients for immediate cystectomy? J. Urol. 177, 75–79; discussion 79 (2007).
Brausi, M. et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur. Urol. 41, 523–531 (2002).
Herr, H. W. Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to Bacillus Calmette-Guerin therapy. J. Urol. 174, 2134–2137 (2005).
Divrik, R. T., Yildirim, U., Zorlu, F. & Ozen, H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J. Urol. 175, 1641–1644 (2006).
Divrik, R. T., Sahin, A. F., Yildirim, U., Altok, M. & Zorlu, F. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur. Urol. 58, 185–190 (2010).
Gontero, P. et al. The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/grade 3 bladder cancer treated with bacille Calmette-Guerin. BJU Int. 118, 44–52 (2016).
Rink, M. et al. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. Eur. Urol. 64, 624–638 (2013).
Bach, T. et al. Optimised photodynamic diagnosis for transurethral resection of the bladder (TURB) in German clinical practice: results of the noninterventional study OPTIC III. World J. Urol. 35, 737–744 (2017).
Gallagher, K. M. et al. ‘Real-life experience’: recurrence rate at 3 years with Hexvix® photodynamic diagnosis-assisted TURBT compared with good quality white light TURBT in new NMIBC-a prospective controlled study. World J. Urol. 35, 1871–1877 (2017).
Herr, H. W. & Donat, S. M. Quality control in transurethral resection of bladder tumours. BJU Int. 102, 1242–1246 (2008).
Naito, S. et al. The Clinical Research Office of the Endourological Society (CROES) multicentre randomised trial of narrow band imaging-assisted transurethral resection of bladder tumour (TURBT) versus conventional white light imaging-assisted TURBT in primary non-muscle-invasive bladder cancer patients: trial protocol and 1-year results. Eur. Urol. 70, 506–515 (2016).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Herr, H. W. et al. Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J. Clin. Oncol. 6, 1450–1455 (1988).
Lamm, D. L. Long-term results of intravesical therapy for superficial bladder cancer. Urol. Clin. North Am. 19, 573–580 (1992).
Lamm, D. L. et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guerin for transitional-cell carcinoma of the bladder. N. Engl. J. Med. 325, 1205–1209 (1991).
Lamm, D. L. et al. Randomized intergroup comparison of Bacillus Calmette-Guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a southwest oncology group study. Urol. Oncol. 1, 119–126 (1995).
Pawinski, A. et al. A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. J. Urol. 156, 1934–1940; discussion 1940–1931 (1996).
Sylvester, R. J., van der, M. A. & Lamm, D. L. Intravesical Bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol. 168, 1964–1970 (2002).
Hemdan, T. et al. 5-year outcome of a randomized prospective study comparing Bacillus Calmette-Guerin with epirubicin and interferon-α2b in patients with T1 bladder cancer. J. Urol. 191, 1244–1249 (2014).
Novotny, V. et al. Impact of adjuvant intravesical Bacillus Calmette-Guerin treatment on patients with high-grade T1 bladder cancer. Urol. Int. 96, 136–141 (2016).
Lamm, D. L. et al. Maintenance Bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J. Urol. 163, 1124–1129 (2000).
Han, R. F. & Pan, J. G. Can intravesical Bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 67, 1216–1223 (2006).
Spiess, P. E. et al. Bladder cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 15, 1240–1267 (2017).
Martinez-Pineiro, L. et al. Maintenance therapy with 3-monthly Bacillus Calmette-Guerin for 3 years is not superior to standard induction therapy in high-risk non-muscle-invasive urothelial bladder carcinoma: final results of randomised CUETO study 98013. Eur. Urol. 68, 256–262 (2015).
Oddens, J. et al. Final results of an EORTC-GU cancers group randomized study of maintenance Bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur. Urol. 63, 462–472 (2013).
Kamat, A. M. et al. Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat. Rev. Urol. 12, 225–235 (2015).
Lerner, S. P. et al. Clarification of bladder cancer disease states following treatment of patients with intravesical BCG. Bladder Cancer 1, 29–30 (2015).
Desai, N. B. et al. Genomic characterization of response to chemoradiation in urothelial bladder cancer. Cancer 122, 3715–3723 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03274284 (2017).
Canter, D. et al. Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: a SEER database analysis. Urol. Oncol. 31, 866–870 (2013).
Fritsche, H. M. et al. Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. Eur. Urol. 57, 300–309 (2010).
Oughton, J. B. et al. Radical cystectomy (bladder removal) against intravesical BCG immunotherapy for high-risk non-muscle invasive bladder cancer (BRAVO): a protocol for a randomised controlled feasibility study. BMJ Open 7, e017913 (2017).
Hedegaard, J. et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 30, 27–42 (2016).
Dyrskjot, L. et al. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 64, 4040–4048 (2004).
Dyrskjot, L. et al. A molecular signature in superficial bladder carcinoma predicts clinical outcome. Clin. Cancer Res. 11, 4029–4036 (2005).
Dyrskjot, L. et al. Prognostic impact of a 12-gene progression score in non-muscle-invasive bladder cancer: a prospective multicentre validation study. Eur. Urol. 72, 461–469 (2017).
Patschan, O. et al. A molecular pathologic framework for risk stratification of stage T1 urothelial carcinoma. Eur. Urol. 68, 824–832; discussion 835–826 (2015).
Pietzak, E. J. et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur. Urol. 72, 952–959 (2017).
Meeks, J. J. et al. Genomic characterization of high-risk non-muscle invasive bladder cancer. Oncotarget 7, 75176–75184 (2016).
Kamat, A. M. et al. Cytokine panel for response to intravesical therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to Bacillus Calmette-Guerin. Eur. Urol. 69, 197–200 (2016).
Kamat, A. M. et al. Predicting response to intravesical Bacillus Calmette-Guerin immunotherapy: are we there yet? A systematic review. Eur. Urol. 73, 738–748 (2017).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet. Oncol. 18, 1483–1492 (2017).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Acknowledgements
The authors would like to acknowledge M. K. Keeter for helpful discussions of the manuscript. J.J.M. is supported by a VA Merit Award (BX003692-01) and a SEED Award from the Hope Foundation.
Reviewer information
Nature Reviews Urology thanks L. Dyrskjot, B. W. G. van Rhijn and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Competing interests
J.J.M. is a consultant for Merck, AstraZeneca and Ferring, and receives research funding from Abbvie, Tesaro, NextCure and Epizyme. B.J. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Non-muscle-invasive bladder cancer
-
(NMIBC). Tumour stages Ta, Tis and T1, which show no evidence of muscle invasion (stage T2).
- Muscle-invasive bladder cancer
-
(MIBC). Tumour stage ≥T2, with evidence of muscle invasion.
- T1 high-grade bladder cancer
-
(T1HG bladder cancer). Following the 2004 International Society of Urological Pathology (ISUP) change in pathology classification, T1HG included some grade G2 and all G3.
- Lymphovascular invasion
-
(LVI). The presence of tumour cells in the lymphatic or vascular channels, usually identified by CD31 or CD34 immunostaining.
- T1 low-grade bladder cancer
-
(T1LG bladder cancer). Following the 2004 International Society of Urological Pathology (ISUP) change in pathology classification, T1LG included grades G1 and some G2.
- Nested variant
-
A variant histology in which the tumour has a benign appearance, simulating von Brunn’s nests, and invades the lamina propria or deeper.
- Papillary bladder cancer
-
Describes a cytoarchitecture of non-muscle-invasive bladder cancer with a central fibrovascular core surrounded by epithelial cells, in contrast with the ‘flat’ cancer found in CIS and sessile invasive tumours.
- Enhanced cystoscopy
-
Cystoscopy with blue light or narrow band imaging.
- Mapping biopsies
-
Small biopsies performed of normal-looking tissue to identify occult carcinoma in situ.
Rights and permissions
About this article
Cite this article
Jordan, B., Meeks, J.J. T1 bladder cancer: current considerations for diagnosis and management. Nat Rev Urol 16, 23–34 (2019). https://doi.org/10.1038/s41585-018-0105-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-018-0105-y
This article is cited by
-
Assessing the prognostic impact of prostatic urethra involvement and developing a nomogram for T1 stage bladder cancer
BMC Urology (2023)
-
Comparative proteomics analysis in different stages of urothelial bladder cancer for identification of potential biomarkers: highlighted role for antioxidant activity
Clinical Proteomics (2023)
-
CD44 is a potential immunotherapeutic target and affects macrophage infiltration leading to poor prognosis
Scientific Reports (2023)
-
In vivo detection of circulating tumor cells predicts high-risk features in patients with bladder cancer
Medical Oncology (2023)
-
A novel model based on disulfidptosis-related genes to predict prognosis and therapy of bladder urothelial carcinoma
Journal of Cancer Research and Clinical Oncology (2023)