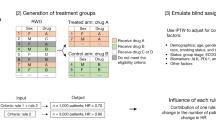
Abstract
Well-designed clinical trials in urological oncology help to guide treatment decisions and aid in counselling patients, ultimately serving to improve outcomes. Since the term evidence-based medicine was first used by Gordon Guyatt in 1991, a renewed emphasis on methodology, transparent trial design and study reporting has helped to improve clinical research and in turn, the landscape of medical literature. Novel clinical trial designs (including multi-arm, multistage trials, basket and umbrella studies and research from big data sources, such as electronic health records, administrative claims databases and quality monitoring registries) are well suited to advance innovation in urological oncology. Existing urological clinical trials are often limited by small numbers, are statistically underpowered and many face difficulties with accrual. Thus, efforts to improve trial design are of considerable importance. The development and use of standard outcome sets and adherence to reporting guidelines offer researchers the opportunity to guide value-oriented care, minimize research waste and efficiently identify solutions to the unanswered questions in urology cancer care.
Key points
-
Innovations in trial design, such as the multi-arm, multistage (MAMS) study type as well as basket and umbrella trial designs, are being used to investigate therapies for urological cancers in a cost-efficient and rapid manner.
-
Urologists should become familiar with the strengths and limitations of big data sources, such as information gathered from systems including electronic health records, administrative claims databases, prescription monitoring registries, whole-genome sequencing and social media.
-
Standard (or core) outcome sets are agreed sets of standardized outcomes that should be measured and reported as a minimum in all clinical trials in a specific area of health or health care; these are developed in conjunction with a panel of treatment experts and patient advocates. Their development and adoption can improve the utility of clinical trial data.
-
Familiarity and adherence to reporting guidelines such as CONSORT and STARD by trial designers, researchers, publishers and users of the medical literature can help to standardize and improve the transparency of trial reporting.
-
Future trials in urological oncology should focus on opportunities to fill gaps within areas of existing guideline deficiency, including areas where current clinical guidance is driven primarily by retrospective observational data or expert opinion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hopewell, S., Dutton, S., Yu, L. M., Chan, A. W. & Altman, D. G. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ 340, c723 (2010).
Carthon, B. C. & Antonarakis, E. S. The STAMPEDE trial: paradigm-changing data through innovative trial design. Transl Cancer Res. 5, S485–S490 (2016).
Parmar, M. K. B. et al. Speeding up the evaluation of new agents in cancer. J. Natl Cancer Inst. 100, 1204–1214 (2008). Provides an overview of the MAMS trial design with a comprehensive overview of its methodology, strengths and limitations.
Pariser, A. R., Robb, M. & Sherman, R. E. Expedited programs for drug development and approval. Expert Opin. Orphan Drugs 1, 507–510 (2013).
US Food and Drug Administration. Food and Drug Administration Safety and Innovation Act (FDASIA) 112–144 (FDA, 2012).
US Food and Drug Administration. Section 506(a) of the Food Drug and Cosmetic Act (FDCA), as added by section 902 of the Food and Drug Administration and Safety and Innovation Act (FDASIA) (FDA, 2018).
Kesselheim, A. & Darrow, J. FDA designations for therapeutics and their impact on drug development and regulatory review outcomes. Clin. Pharmacol. Ther. 97, 29–36 (2015).
Hwang, T. J. et al. Efficacy, safety, and regulatory approval of food and drug administration-designated breakthrough and nonbreakthrough cancer medicines. J. Clin. Oncol. 36, 1805–1812 (2018). An important study that highlighted the fact that despite the earlier regulatory approval of breakthrough-therapy-designated drugs, no evidence exists that these drugs provide improvements in safety or novelty.
Shea, M. et al. Impact of breakthrough therapy designation on cancer drug development. Nat. Rev. Drug Discov. 15, 152–152 (2016).
Krishnamurti, T., Woloshin, S., Schwartz, L. M. & Fischhoff, B. A randomized trial testing US Food and Drug Administration “breakthrough” language. JAMA Intern. Med. 175, 1856 (2015).
European Medicines Agency. Human regulatory. Accelerated assessment (EMA, 2019).
European Medicines Agency. Human regulatory. Conditional marketing authorisation (EMA, 2019).
European Medicines Agency. Human regulatory. PRIME: priority medicines (EMA, 2019).
Ellis, L. M. et al. American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J. Clin. Oncol. 32, 1277–1280 (2014). A highly cited and well-written summary of the American Society of Clinical Oncology Cancer Research Committee working group deliberations establishing end points that are clinically meaningful to patients. Although focused on pancreas, breast, lung and colon cancers, this reference provides a blueprint for similar efforts to be undertaken by trialists in urological oncology.
US Food and Drug Administration. Adaptive designs for clinical trials of drugs and biologics, guidance for industry (draft guidance) (FDA, 2018).
Proschan, M. A. Sample size re-estimation in clinical trials. Biom. J. 51, 348–357 (2009).
Mehta, C. R. & Patel, N. R. Adaptive, group sequential and decision theoretic approaches to sample size determination. Stat. Med. 25, 3250–3269 (2006).
Simon, N. & Simon, R. Adaptive enrichment designs for clinical trials. Biostatistics 14, 613–625 (2013).
Bretz, F., Schmidli, H., König, F., Racine, A. & Maurer, W. Confirmatory seamless phase II/III clinical trials with hypotheses selection at interim: general concepts. Biom. J. 48, 623–634 (2006).
Sydes, M. R. et al. Flexible trial design in practice — stopping arms for lack-of-benefit and adding research arms mid-trial in STAMPEDE: a multi-arm multi-stage randomized controlled trial. Trials 13, 168 (2012). A review of both practical and statistical issues encountered by the STAMPEDE authors in implementing MAMS methodology, with an overview of how these issues were addressed.
Schaid, D. J., Wieand, S. & Therneau, T. M. Optimal two-stage screening designs for survival comparisons. Biometrika 77, 507–513 (1990).
Royston, P., Parmar, M. K. B. & Qian, W. Novel designs for multi-arm clinical trials with survival outcomes with an application in ovarian cancer. Stat. Med. 22, 2239–2256 (2003).
Sydes, M. R. et al. Issues in applying multi-arm multi-stage methodology to a clinical trial in prostate cancer: the MRC STAMPEDE trial. Trials 10, 39 (2009).
Medical Research Council. STAMPEDE. Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (MRC, 2018).
James, N. D. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016). Established docetaxel chemotherapy as standard of care along with androgen-deprivation therapy for men with metastatic prostate cancer.
James, N. D. et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med. 377, 338–351 (2017). Established abiraterone and prednisolone as a standard of care with androgen-deprivation therapy for men with locally advanced or metastatic prostate cancer.
Parker, C. C. et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 392, 2353–2366 (2018).
Gillessen, S. et al. Repurposing metformin as therapy for prostate cancer within the STAMPEDE trial platform. Eur. Urol. 70, 906–908 (2016).
James, N. D. et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from the STAMPEDE multiarm, multistage, randomised controlled trial. Lancet Oncol. 13, 549–558 (2012).
Lachin, J. M. Introduction to sample size determination and power analysis for clinical trials. Control. Clin. Trials. 2, 93–113 (1981).
Freidlin, B., Korn, E. L., Gray, R. & Martin, A. Multi-arm clinical trials of new agents: some design considerations. Clin. Cancer Res. 14, 4368–4371 (2008).
Wason, J. M. S. & Jaki, T. Optimal design of multi-arm multi-stage trials. Stat. Med. 31, 4269–4279 (2012).
Bratton, D. J., Parmar, M. K. B., Phillips, P. P. J. & Choodari-Oskooei, B. Type I error rates of multi-arm multi-stage clinical trials: strong control and impact of intermediate outcomes. Trials 17, 309 (2016).
Wason, J. M. S., Stecher, L. & Mander, A. P. Correcting for multiple-testing in multi-arm trials: is it necessary and is it done? Trials 15, 364 (2014).
Bland, J. M. & Altman, D. G. Multiple significance tests: the Bonferroni method. BMJ 310, 170–170 (1995).
Moher, D. et al. CONSORT 2010 Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340, c869–c869 (2010). A key reference summarizing the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement, with explanation of CONSORT principles to guide investigators when preparing trial manuscripts.
Sweeney, C. J. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
Bratton D. Design Issues and Extensions of Multi-arm Multi-stage Clinical Trials. Thesis, UCL (2015).
Jitlal, M., Khan, I., Lee, S. M. & Hackshaw, A. Stopping clinical trials early for futility: retrospective analysis of several randomised clinical studies. Br. J. Cancer 107, 910–917 (2012).
Renfro, L. A. & Sargent, D. J. Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples. Ann. Oncol. J. Eur. Soc. Med. Oncol. 28, 34–43 (2017). Detailed overview of basket and umbrella trial designs, including definitions, examples, and a review of their strengths and limitations in practice.
Pili, R. et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 29, 4022–4028 (2011).
Kelly, W. K. et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 30, 1534–1540 (2012).
Seiler, R. et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur. Urol. 72(4), 544–554 (2017).
Plimack, E. R. et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 18, 212–220 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02152254 (2019).
Hyman, D. M. et al. Precision medicine at Memorial Sloan Kettering Cancer Center: clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov. Today 20, 1422–1428 (2015).
Lopez-Chavez, A. et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J. Clin. Oncol. 33, 1000–1007 (2015).
Hazim, A. & Prasad, V. A pooled analysis of published, basket trials in cancer medicine. Eur. J. Cancer 101, 244–250 (2018).
Alden, R. S., Mandrekar, S. J. & Oxnard, G. R. Designing a definitive trial for adjuvant targeted therapy in genotype defined lung cancer: the ALCHEMIST trials. Chin. Clin. Oncol. 4, 37 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03385655 (2019).
Simon, R. Critical review of umbrella, basket, and platform designs for oncology clinical trials. Clin. Pharmacol. Ther. 102, 934–941 (2017).
Cunanan, K. M., Iasonos, A., Shen, R., Begg, C. B. & Gönen, M. An efficient basket trial design. Stat. Med. 36, 1568–1579 (2017).
Murdoch, T. B. & Detsky, A. S. The inevitable application of big data to health care. JAMA 309, 1351 (2013).
Ghani, K. R., Zheng, K., Wei, J. T. & Friedman, C. P. Harnessing big data for health care and research: are urologists ready? Eur. Urol. 66, 975–977 (2014).
Auffray, C. et al. Making sense of big data in health research: towards an EU action plan. Genome Med. 8, 71 (2016).
ELIXIR. A distributed infrastructure for life-science information. elixir https://elixir-europe.org/ (2019).
Yu, J. B., Gross, C. P., Wilson, L. D. & Smith, B. D. NCI SEER public-use data: applications and limitations in oncology research. Oncology 23, 288–295 (2009).
Giordano, S. H. et al. Limits of observational data in determining outcomes from cancer therapy. Cancer 112, 2456–2466 (2008). This paper highlights the limitations of observational data, particularly those derived from claims registries such as SEER.
Soni, P. D. et al. Comparison of population-based observational studies with randomized trials in oncology. J. Clin. Oncol. 37, 1209–1216 (2019).
Schlomer, B. J. & Copp, H. L. Secondary data analysis of large data sets in urology: successes and errors to avoid. J. Urol. 191, 587–596 (2014).
Howerton, M. W. et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer 109, 465–476 (2007). A systematic review that highlights the role that providers and caregivers have in adversely influencing the recruitment of under-represented groups to clinical trials.
TSSAHA Panel. Final report of the Tuskegee Syphilis Study Ad Hoc Advisory Panel. https://biotech.law.lsu.edu/cphl/history/reports/tuskegee/report1.pdf (1973).
Green, B. L., Maisiak, R., Wang, M. Q., Britt, M. F. & Ebeling, N. Participation in health education, health promotion, and health research by African Americans: effects of the Tuskegee Syphilis Experiment. J. Health Educ. 28, 196–201 (1997).
Simon, M. S. et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J. Clin. Oncol. 22, 2046–2052 (2004).
Ni, Y. et al. Increasing the efficiency of trial-patient matching: automated clinical trial eligibility pre-screening for pediatric oncology patients. BMC Med. Inf. Decis. Mak. 15, 28 (2015).
Murff, H. J. et al. Automated identification of postoperative complications within an electronic medical record using natural language processing. JAMA 306, 848–855 (2011).
Choi, W. et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014).
Jeong, I. G. et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA 318, 1561 (2017).
Cooperberg, M. R., Fang, R., Schlossberg, S., Wolf, J. S. & Clemens, J. Q. The AUA quality registry: engaging stakeholders to improve the quality of care for patients with prostate cancer. Urol. Pract. 4, 30–35 (2017).
Schwartz, D. & Lellouch, J. Explanatory and pragmatic attitudes in therapeutical trials. J. Chronic. Dis. 20, 637–648 (1967).
Thorpe, K. E. et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 180, E47–E57 (2009).
Barocas, D. A. et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: the CEASAR study. J. Comp. Eff. Res. 2, 445–460 (2013).
Chalmers, I. & Glasziou, P. Avoidable waste in the production and reporting of research evidence. Lancet 374, 86–89 (2009). An important editorial regarding the concept of 'research waste', and an exploration of the various stages of research design and development that can contribute to waste production.
Stuckler, D., King, L., Robinson, H. & McKee, M. WHO’s budgetary allocations and burden of disease: a comparative analysis. Lancet 372, 1563–1569 (2008).
Tallon, D., Chard, J. & Dieppe, P. Relation between agendas of the research community and the research consumer. Lancet 355, 2037–2040 (2000).
Williamson, P. R. et al. Developing core outcome sets for clinical trials: issues to consider. Trials 13, 132 (2012).
Martin, N. E. et al. Defining a standard set of patient-centered outcomes for men with localized prostate cancer. Eur. Urol. 67, 460–467 (2015). An example of a standard (core) outcome set for prostate cancer, as defined by the ICHOM working group.
Foust-Wright, C. et al. Development of a core set of outcome measures for OAB treatment. Int. Urogynecol. J. 28, 1785–1793 (2017).
Ackerman, I. N., Cavka, B., Lippa, J. & Bucknill, A. The feasibility of implementing the ICHOM standard set for hip and knee osteoarthritis: a mixed-methods evaluation in public and private hospital settings. J. Patient Rep. Outcomes 2, 32 (2017).
Curran, G. M., Bauer, M., Mittman, B., Pyne, J. M. & Stetler, C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med. Care 50, 217–226 (2012).
Bossuyt, P. M. et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann. Intern. Med. 138, W1–W12 (2003). Similar to CONSORT, the STARD statement provides a checklist for ensuring transparent reporting of diagnostic accuracy test studies.
Narayan, V. M., Cone, E. B., Smith, D., Scales, C. D. & Dahm, P. Improved reporting of randomized controlled trials in the urologic literature. Eur. Urol. 70, 1044–1049 (2016).
Turner, L. et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 11, MR000030 (2012).
Smith, D. W., Gandhi, S. & Dahm, P. The reporting quality of studies of diagnostic accuracy in the urologic literature. World J. Urol. 37, 969–974 (2019).
Narayan V. M. A critical appraisal of biomarkers in prostate cancer. World J. Urol. https://doi.org/10.1007/s00345-019-02759-x (2019).
Author information
Authors and Affiliations
Contributions
Both authors made substantial contribution to the discussion of content, wrote the article and reviewed and edited the article before submission. V.M.N. researched data for the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks K. Cunanan, T. Skolarus and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Type I error
-
The incorrect rejection of a true null hypothesis, resulting in a false-positive conclusion.
- Family-wise error
-
The occurrence of making a type I (false-positive) error, and doing so within the context of multiple testing (for example, when more than one hypothesis is being tested).
- Natural language processing software
-
Software that is able to impute discrete data from free-text information entered using human syntax; for example, natural language processing software could be used to scan a patient’s progress note and identify the patient’s age, demographic information, current symptoms and treatment plan.
- Meaningful use reporting initiatives
-
US government standards for electronic medical record use, specifically focused on promoting the adoption of electronic medical records, exchange of patient information electronically and standardizing methods for electronic prescribing, care coordination, case reporting and billing.
- Cohort inception tool
-
A method of identifying study groups (cohorts), usually used within the contexts of claims or quality registries.
Rights and permissions
About this article
Cite this article
Narayan, V.M., Dahm, P. The future of clinical trials in urological oncology. Nat Rev Urol 16, 722–733 (2019). https://doi.org/10.1038/s41585-019-0243-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-019-0243-x
This article is cited by
-
Cytoreductive Nephrectomy in the Management of Metastatic Renal Cell Carcinoma: Is There Still a Debate?
Current Urology Reports (2021)
-
Stability and anti-proliferative properties of biologically active compounds extracted from Cistus L. after sterilization treatments
Scientific Reports (2020)
-
Competitive glucose metabolism as a target to boost bladder cancer immunotherapy
Nature Reviews Urology (2020)