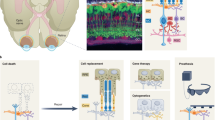
Abstract
Restoring vision to the blind by retinal repair has been a dream of medicine for centuries, and the first successful procedures have recently been performed. Although we are still far from the restoration of high-resolution vision, step-by-step developments are overcoming crucial bottlenecks in therapy development and have enabled the restoration of some visual function in patients with specific blindness-causing diseases. Here, we discuss the current state of vision restoration and the problems related to retinal repair. We describe new model systems and translational technologies, as well as the clinical conditions in which new methods may help to combat blindness.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
The Lasker Foundation. Restoring Vision to the Blind http://www.laskerfoundation.org/new-noteworthy/articles/restoring-vision-blind/ (2015).
Olson, R. J. Cataract surgery from 1918 to the present and future—just imagine! Am. J. Ophthalmol. 185, 10–13 (2017).
Jager, R. D., Mieler, W. F. & Miller, J. W. Age-related macular degeneration. N. Engl. J. Med. 358, 2606–2617 (2008).
Hodgson, N. et al. Economic and quality of life benefits of anti-VEGF therapy. Mol. Pharm. 13, 2877–2880 (2016).
Finger, R. P., Guymer, R. H., Gillies, M. C. & Keeffe, J. E. The impact of anti-vascular endothelial growth factor treatment on quality of life in neovascular age-related macular degeneration. Ophthalmology 121, 1246–1251 (2014).
Essue, B. M. et al. A multicenter prospective cohort study of quality of life and economic outcomes after cataract surgery in Vietnam: the VISIONARY study. Ophthalmology 121, 2138–2146 (2014).
Lamoureux, E. L., Fenwick, E., Pesudovs, K. & Tan, D. The impact of cataract surgery on quality of life. Curr. Opin. Ophthalmol. 22, 19–27 (2011).
WHO. Visual Impairment and Blindness http://www.who.int/mediacentre/factsheets/fs282/en/ (2017).
Bainbridge, J. W. B. et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 (2008).
Hauswirth, W. W. et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 (2008).
Maguire, A. M. et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).This study led to the first FDA-approved AAV gene therapy.
Russell, S. et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017).The first FDA-approved AAV gene therapy.
Ashtari, M. et al. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J. Clin. Invest. 121, 2160–2168 (2011).
Ashtari, M. et al. Plasticity of the human visual system after retinal gene therapy in patients with Leber’s congenital amaurosis. Sci. Transl. Med. 7, 296ra110 (2015).
Ledford, H. FDA advisers back gene therapy for rare form of blindness. Nature 550, 314 (2017).
MacLaren, R. E. et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 383, 1129–1137 (2014).
Edwards, T. L. et al. Visual acuity after retinal gene therapy for choroideremia. N. Engl. J. Med. 374, 1996–1998 (2016).
Humayun, M. S. et al. Visual perception elicited by electrical stimulation of retina in blind humans. Arch. Ophthalmol. 114, 40–46 (1996).This work led to the development of epiretinal implants.
Mills, J. O., Jalil, A. & Stanga, P. E. Electronic retinal implants and artificial vision: journey and present. Eye 31, 1383–1398 (2017).
da Cruz, L. et al. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology 123, 2248–2254 (2016).
Schwartz, S. D. et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516 (2015).
Mandai, M. et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 (2017).
Tanna, P., Strauss, R. W., Fujinami, K. & Michaelides, M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 101, 25–30 (2017).
Daley, G. Q. Polar extremes in the clinical use of stem cells. N. Engl. J. Med. 376, 1075–1077 (2017).
Espinosa, J. S. & Stryker, M. P. Development and plasticity of the primary visual cortex. Neuron 75, 230–249 (2012).
Ganesh, S. et al. Results of late surgical intervention in children with early-onset bilateral cataracts. Br. J. Ophthalmol. 98, 1424–1428 (2014).
Kalia, A. et al. Development of pattern vision following early and extended blindness. Proc. Natl Acad. Sci. USA 111, 2035–2039 (2014).
Sinha, P., Chatterjee, G., Gandhi, T. & Kalia, A. Restoring vision through “Project Prakash”: the opportunities for merging science and service. PLoS Biol. 11, e1001741 (2013).
Wan, J. & Goldman, D. Retina regeneration in zebrafish. Curr. Opin. Genet. Dev. 40, 41–47 (2016).
Azeredo da Silveira, R. & Roska, B. Cell types, circuits, computation. Curr. Opin. Neurobiol. 21, 664–671 (2011).
Masland, R. H. The neuronal organization of the retina. Neuron 76, 266–280 (2012).
Zeng, H. & Sanes, J. R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci. 18, 530–546 (2017).
Seung, H. S. & Sümbül, U. Neuronal cell types and connectivity: lessons from the retina. Neuron 83, 1262–1272 (2014).
Weinmann, J. & Grimm, D. Next-generation AAV vectors for clinical use: an ever-accelerating race. Virus Genes 53, 707–713 (2017).
Santos-Ferreira, T. F., Borsch, O. & Ader, M. Rebuilding the missing part—a review on photoreceptor transplantation. Front. Syst. Neurosci. 10, 105 (2017).
Chalupa, L. M. & Williams, R. W. (eds) Eye, Retina, and Visual System of the Mouse (MIT Press, Cambridge, 2008).
Dacey, D. M. The mosaic of midget ganglion cells in the human retina. J. Neurosci. 13, 5334–5355 (1993).
Kolb, H. in Webvision: The Organization of the Retina and Visual System (eds Kolb, H. et al.) (Univ. Utah Health Sciences Center, Salt Lake City, 1995).
Sahly, I. et al. Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J. Cell Biol. 199, 381–399 (2012).Example of the limitations of mice as a disease model.
Siegert, S. et al. Transcriptional code and disease map for adult retinal cell types. Nat. Neurosci. 15, 487–495 (2012).
Kostic, C. & Arsenijevic, Y. Animal modelling for inherited central vision loss. J. Pathol. 238, 300–310 (2016).
Kolb, H., Fernandez, E. & Nelson, R. (eds) Webvision: The Organization of the Retina and Visual System (Univ. Utah Health Sciences Center, Salt Lake City, 1995).
Seiple, W., Rosen, R. B. & Garcia, P. M. T. Abnormal fixation in individuals with age-related macular degeneration when viewing an image of a face. Optom. Vis. Sci. 90, 45–56 (2013).
Dalkara, D. et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 17, 2096–2102 (2009).
Dalkara, D. et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5, 189ra76 (2013).
Yin, L. et al. Intravitreal injection of AAV2 transduces macaque inner retina. Invest. Ophthalmol. Vis. Sci. 52, 2775–2783 (2011).
Panda-Jonas, S., Jonas, J. B., Jakobczyk, M. & Schneider, U. Retinal photoreceptor count, retinal surface area, and optic disc size in normal human eyes. Ophthalmology 101, 519–523 (1994).
Remtulla, S. & Hallett, P. E. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 25, 21–31 (1985).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).This study led to the development of human retinal organoids.
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Sasai, Y. Grow your own eye: biologists have coaxed cells to form a retina, a step toward growing replacement organs outside the body. Sci. Am. 307, 44–49 (2012).
Ovando-Roche, P., Georgiadis, A., Smith, A. J., Pearson, R. A. & Ali, R. R. Harnessing the potential of human pluripotent stem cells and gene editing for the treatment of retinal degeneration. Curr. Stem Cell Rep. 3, 112–123 (2017).
Kaewkhaw, R. et al. Transcriptome dynamics of developing photoreceptors in three-dimensional retina cultures recapitulates temporal sequence of human cone and rod differentiation revealing cell surface markers and gene networks. Stem Cells 33, 3504–3518 (2015).
Busskamp, V. et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329, 413–417 (2010).Demonstration of optogenetic vision restoration in human retinas.
Fradot, M. et al. Gene therapy in ophthalmology: validation on cultured retinal cells and explants from postmortem human eyes. Hum. Gene Ther. 22, 587–593 (2011).
Dowling, J. E. The Retina (Harvard Univ. Press, Cambridge, 2012)
Izpisua Belmonte, J. C. et al. Brains, genes, and primates. Neuron 86, 617–631 (2015).
Mitchell, J. F. & Leopold, D. A. The marmoset monkey as a model for visual neuroscience. Neurosci. Res. 93, 20–46 (2015).
Sahel, J.-A. & Roska, B. Gene therapy for blindness. Annu. Rev. Neurosci. 36, 467–488 (2013).
Carter, B. J. Adeno-associated virus and the development of adeno-associated virus vectors: a historical perspective. Mol. Ther. 10, 981–989 (2004).
Ali, R. R. et al. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum. Mol. Genet. 5, 591–594 (1996).The first AAV gene transfer to the retina of mice.
Flannery, J. G. et al. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc. Natl Acad. Sci. USA 94, 6916–6921 (1997).
Planul, A. & Dalkara, D. Vectors and gene delivery to the retina. Annu. Rev. Vis. Sci. 3, 121–140 (2017).
Madigan, V. J. & Asokan, A. Engineering AAV receptor footprints for gene therapy. Curr. Opin. Virol. 18, 89–96 (2016).
Chaffiol, A. et al. A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol. Ther. 25, 2546–2560 (2017).
Jacobson, S. G. et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 130, 9–24 (2012).
Wang, S. et al. Non-invasive, focused ultrasound-facilitated gene delivery for optogenetics. Sci. Rep. 7, 39955 (2017).
Wan, C., Li, F. & Li, H. Gene therapy for ocular diseases meditated by ultrasound and microbubbles. Mol. Med. Rep. 12, 4803–4814 (2015).
Touchard, E. et al. Non-viral gene therapy for GDNF production in RCS rat: the crucial role of the plasmid dose. Gene Ther. 19, 886–898 (2012).
MacLaren, R. E. et al. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203–207 (2006).
Pearson, R. A. et al. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103 (2012).
MacLaren, R. E. Cone fusion confusion in photoreceptor transplantation. Stem Cell Investig. 4, 71 (2017).
Singh, M. S. et al. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 7, 13537 (2016).
Pearson, R. A. et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 7, 13029 (2016).
Santos-Ferreira, T. et al. Retinal transplantation of photoreceptors results in donor–host cytoplasmic exchange. Nat. Commun. 7, 13028 (2016).
Hertz, J. et al. Survival and integration of developing and progenitor-derived retinal ganglion cells following transplantation. Cell Transplant. 23, 855–872 (2014).
Venugopalan, P. et al. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 7, 10472 (2016).
Sahel, J. A. et al. Mitogenic effects of excitatory amino acids in the adult rat retina. Exp. Eye Res. 53, 657–664 (1991).
Jorstad, N. L. et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103–107 (2017).
Benowitz, L. & Yin, Y. Rewiring the injured CNS: lessons from the optic nerve. Exp. Neurol. 209, 389–398 (2008).
Lim, J.-H. A. et al. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci. 19, 1073–1084 (2016).
Sun, F. et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480, 372–375 (2011).
Laha, B., Stafford, B. K. & Huberman, A. D. Regenerating optic pathways from the eye to the brain. Science 356, 1031–1034 (2017).
Yue, L., Weiland, J. D., Roska, B. & Humayun, M. S. Retinal stimulation strategies to restore vision: fundamentals and systems. Prog. Retin. Eye Res. 53, 21–47 (2016).
Scholl, H. P. N. et al. Emerging therapies for inherited retinal degeneration. Sci. Transl. Med. 8, 368rv6 (2016).
Stingl, K. et al. Interim results of a multicenter trial with the new electronic subretinal implant Alpha AMS in 15 patients blind from inherited retinal degenerations. Front. Neurosci. 11, 445 (2017).
Stronks, H. C. & Dagnelie, G. The functional performance of the Argus II retinal prosthesis. Expert Rev. Med. Devices 11, 23–30 (2014).
Mullin, E. A company is reviving efforts to make a bionic eye brain implant for the blind. MIT Technol. Rev. https://www.technologyreview.com/s/608844/blind-patients-to-test-bionic-eye-brain-implants/ (2017).
Bi, A. et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50, 23–33 (2006).
Lagali, P. S. et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 11, 667–675 (2008).
Polosukhina, A. et al. Photochemical restoration of visual responses in blind mice. Neuron 75, 271–282 (2012).
Busskamp, V., Picaud, S., Sahel, J. A. & Roska, B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 19, 169–175 (2012).
Berndt, A., Yizhar, O., Gunaydin, L. A., Hegemann, P. & Deisseroth, K. Bi-stable neural state switches. Nat. Neurosci. 12, 229–234 (2009).
Hartong, D. T., Berson, E. L. & Dryja, T. P. Retinitis pigmentosa. Lancet 368, 1795–1809 (2006).
den Hollander, A. I., Roepman, R., Koenekoop, R. K. & Cremers, F. P. M. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 27, 391–419 (2008).
Dobelle, W. H., Mladejovsky, M. G. & Girvin, J. P. Artifical vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science 183, 440–444 (1974).
Bourne, D. et al. Whole-eye transplantation: a look into the past and vision for the future. Eye (Lond.) 31, 179–184 (2017).
Miller, J. W. Beyond VEGF—the Weisenfeld lecture. Invest. Ophthalmol. Vis. Sci. 57, 6911–6918 (2016).
Schwartz, S. D., Tan, G., Hosseini, H. & Nagiel, A. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest. Ophthalmol. Vis. Sci. 57, ORSFc1–ORSFc9 (2016).
Parker, M. A. et al. Test–retest variability of functional and structural parameters in patients with Stargardt disease participating in the SAR422459 gene therapy trial. Transl. Vis. Sci. Technol. 5, 10 (2016).
Byrne, L. C. et al. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J. Clin. Invest. 125, 105–116 (2015).
Léveillard, T. et al. Identification and characterization of rod-derived cone viability factor. Nat. Genet. 36, 755–759 (2004).Discovery of a factor for photoreceptor neuroprotection.
Mohand-Said, S. et al. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc. Natl Acad. Sci. USA 95, 8357–8362 (1998).
Aït-Ali, N. et al. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161, 817–832 (2015).
Venkatesh, A. et al. Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J. Clin. Invest. 125, 1446–1458 (2015).
Punzo, C., Kornacker, K. & Cepko, C. L. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat. Neurosci. 12, 44–52 (2009).Discovery of a factor for photoreceptor neuroprotection.
Xiong, W., MacColl Garfinkel, A. E., Li, Y., Benowitz, L. I. & Cepko, C. L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J. Clin. Invest. 125, 1433–1445 (2015).
Cepko, C. & Punzo, C. Cell metabolism: sugar for sight. Nature 522, 428–429 (2015).
Krol, J. & Roska, B. Rods feed cones to keep them alive. Cell 161, 706–708 (2015).
Léveillard, T. & Sahel, J.-A. Metabolic and redox signaling in the retina. Cell. Mol. Life Sci. 74, 3649–3665 (2016).
Campochiaro, P. A. et al. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid. Redox Signal. 23, 643–648 (2015).
Kayser, S. et al. Reduced central retinal artery blood flow is related to impaired central visual function in retinitis pigmentosa patients. Curr. Eye Res. 42, 1503–1510 (2017).
Chaikin, L., Kashiwa, K., Bennet, M., Papastergiou, G. & Gregory, W. Microcurrent stimulation in the treatment of dry and wet macular degeneration. Clin. Ophthalmol. 9, 2345–2353 (2015).
Schatz, A. et al. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled follow-up study over 1 year. Invest. Ophthalmol. Vis. Sci. 58, 257–269 (2017).
Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 119, 1417–1436 (2001).
Jurkute, N. & Yu-Wai-Man, P. Leber hereditary optic neuropathy: bridging the translational gap. Curr. Opin. Ophthalmol. 28, 403–409 (2017).
Smirnakis, S. M. Probing human visual deficits with functional magnetic resonance imaging. Annu. Rev. Vis. Sci. 2, 171–195 (2016).
Bainbridge, J. W. B. et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 372, 1887–1897 (2015).
Jacobson, S. G. et al. Improvement and decline in vision with gene therapy in childhood blindness. N. Engl. J. Med. 372, 1920–1926 (2015).
Rizzo, J. F. III & Ayton, L. N. Psychophysical testing of visual prosthetic devices: a call to establish a multi-national joint task force. J. Neural Eng. 11, 020301 (2014).
Finger, R. P. et al. Developing the impact of Vision Impairment-Very Low Vision (IVI-VLV) questionnaire as part of the LoVADA protocol. Invest. Ophthalmol. Vis. Sci. 55, 6150–6158 (2014).
Jeter, P. E., Rozanski, C., Massof, R., Adeyemo, O. & Dagnelie, G. Development of the Ultra-Low Vision Visual Functioning Questionnaire (ULV-VFQ). Transl. Vis. Sci. Technol. 6, 11 (2017).
Authié, C. N., Berthoz, A., Sahel, J.-A. & Safran, A. B. Adaptive gaze strategies for locomotion with constricted visual field. Front. Hum. Neurosci. 11, 387 (2017).
Cattaneo, Z. & Vecchi, T. Blind Vision (MIT Press, Cambridge, 2011).
Irvine, D. et al. Tablet and smartphone accessibility features in the low vision rehabilitation. Neuroophthalmology 38, 53–59 (2014).
Robinson, J. L., Braimah Avery, V., Chun, R., Pusateri, G. & Jay, W. M. Usage of accessibility options for the iPhone and iPad in a visually impaired population. Semin. Ophthalmol. 32, 163–171 (2017).
Alter, C. Facebook is developing technology that can describe pictures to blind people. Time http://time.com/4099204/facebook-artificial-intelligence-blind-pictures/ (2015).
Striem-Amit, E., Guendelman, M. & Amedi, A. ‘Visual’ acuity of the congenitally blind using visual-to-auditory sensory substitution. PLoS One 7, e33136 (2012).
Striem-Amit, E., Cohen, L., Dehaene, S. & Amedi, A. Reading with sounds: sensory substitution selectively activates the visual word form area in the blind. Neuron 76, 640–652 (2012).
Thaler, L. & Goodale, M. A. Echolocation in humans: an overview. Wiley Interdiscip. Rev. Cogn. Sci. 7, 382–393 (2016).
Köberlein, J., Beifus, K., Schaffert, C. & Finger, R. P. The economic burden of visual impairment and blindness: a systematic review. BMJ Open 3, e003471 (2013).
Romanov, R. A. et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci. 20, 176–188 (2017).
Acknowledgements
We thank S. Trenholm for discussions, comments and advice on the manuscript; A. Drinnenberg, D. Dalkara, S. Picaud, I. Audo, K. Marazova, S. Oakeley and P. King for comments on the manuscript; and P. Maloca for images for Fig. 1, A. Drinnenberg for drawings for Fig. 2, and V. Juvin (http://www.sciartwork.com) for images for Fig. 3.
Reviewer information
Nature thanks G. Dagnelie, J. Demb and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
B.R. and J.A.-S. discussed and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.A.-S. has financial interests in GenSight Biologics, Chronocam, Chronolife, Pixium Vision, Tilak Healthcare, and Sparing Vision.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roska, B., Sahel, JA. Restoring vision. Nature 557, 359–367 (2018). https://doi.org/10.1038/s41586-018-0076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0076-4
This article is cited by
-
Attitudes of potential recipients toward emerging visual prosthesis technologies
Scientific Reports (2023)
-
MAP4Ks inhibition promotes retinal neuron regeneration from Müller glia in adult mice
npj Regenerative Medicine (2023)
-
Assessment of visual function in blind mice and monkeys with subretinally implanted nanowire arrays as artificial photoreceptors
Nature Biomedical Engineering (2023)
-
Structural and functional distinctions of co-resident microglia and monocyte-derived macrophages after retinal degeneration
Journal of Neuroinflammation (2022)
-
Flexible ultrasound-induced retinal stimulating piezo-arrays for biomimetic visual prostheses
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.