Abstract
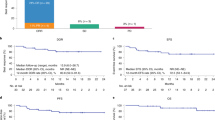
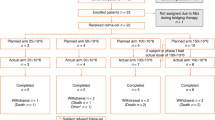
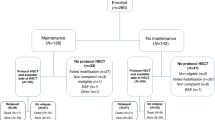
Tisagenlecleucel is an autologous anti-CD19 chimeric antigen receptor-T cell therapy with clinically meaningful outcomes demonstrated in patients with relapsed/refractory (r/r) B-cell lymphoma. In a previous pilot study of tisagenlecleucel in r/r follicular lymphoma (FL), 71% of patients achieved a complete response (CR). Here we report the primary, prespecified interim analysis of the ELARA phase 2 multinational trial of tisagenlecleucel in adults with r/r FL after two or more treatment lines or who relapsed after autologous stem cell transplant (no. NCT03568461). The primary endpoint was CR rate (CRR). Secondary endpoints included overall response rate (ORR), duration of response, progression-free survival, overall survival, pharmacokinetics and safety. As of 29 March 2021, 97/98 enrolled patients received tisagenlecleucel (median follow-up, 16.59 months; interquartile range, 13.8–20.21). The primary endpoint was met. In the efficacy set (n = 94), CRR was 69.1% (95% confidence interval, 58.8–78.3) and ORR 86.2% (95% confidence interval, 77.5–92.4). Within 8 weeks of infusion, rates of cytokine release syndrome were 48.5% (grade ≥3, 0%), neurological events 37.1% (grade ≥3, 3%) and immune effector cell-associated neurotoxicity syndrome (ICANS) 4.1% (grade ≥3, 1%) in the safety set (n = 97), with no treatment-related deaths. Tisagenlecleucel is safe and effective in extensively pretreated r/r FL, including in high-risk patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this analysis are available within the article and its appendix. Novartis is committed to sharing, with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. Availability of trial data is according to the criteria and process described at www.clinicalstudydatarequest.com.
References
Swerdlow, S. H. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127, 2375–2390 (2016).
Marcus, R. et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 377, 1331–1344 (2017).
Lansigan, F. et al. The prognostic significance of PFS24 in follicular lymphoma following firstline immunotherapy: a combined analysis of 3 CALGB trials. Cancer Med. 8, 165–173 (2019).
Salles, G. et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 377, 42–51 (2011).
Rivas‐Delgado, A. et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br. J. Haematol. 184, 753–759 (2019).
Link, B. K. et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br. J. Haematol. 184, 660–663 (2019).
Casulo, C. et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J. Clin. Oncol. 33, 2516–2522 (2015).
Sarkozy, C. et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: a pooled analysis of French and US cohorts. J. Clin. Oncol. 37, 144–152 (2019).
Gopal, A. K. et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 370, 1008–1018 (2014).
Salles, G. et al. Efficacy and safety of idelalisib in patients with relapsed, rituximab-and alkylating agent-refractory follicular lymphoma: a subgroup analysis of a phase 2 study. Haematologica 102, e156–e159 (2017).
Dreyling, M. et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J. Clin. Oncol. 35, 3898–3905 (2017).
Zinzani, P. L. et al. DYNAMO: A phase 2 study demonstrating the clinical activity of duvelisib in patients with double-refractory follicular lymphoma. https://library.ehaweb.org/eha/2017/22nd/182064/pier.luigi.zinzani.dynamo.a.phase.2.study.demonstrating.the.clinical.activity.html (2017).
Ukoniq; https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/213176Orig1s000,%20213176Orig2s000ltr.pdf (2021).
US Food and Drug Administration. FDA approves lenalidomide for follicular and marginal zone lymphoma. May 2019; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenalidomide-follicular-and-marginal-zone-lymphoma#:~:text=On%20May%2028%2C%202019%2C%20the,marginal%20zone%20lymphoma%20(MZL) (2021).
Leonard, J. P. et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J. Clin. Oncol. 37, 1188–1199 (2019).
Rummel, M. J. et al. MAGNIFY: phase IIIb interim analysis of induction lenalidomide + rituximab (R2) followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. In 25th Congress of the European Hematology Association (EHA) Poster EP1161 (2020).
Bodor, C. et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 122, 3165–3168 (2013).
Morschhauser, F. et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 21, 1433–1442 (2020).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
US Food and Drug Administration. FDA grants accelerated approval to axicabtagene ciloleucel for relapsed or refractory follicular lymphoma; https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-axicabtagene-ciloleucel-relapsed-or-refractory-follicular-lymphoma (2021).
Chong, E. A. et al. CD19-directed CAR T cell therapy (CTL019) for relapsed/refractory diffuse large B-cell and follicular lymphomas: four year outcomes. In 15th International Conference on Malignant Lymphoma Abstract 090 (2019).
Chong, E. A., Ruella, M. & Schuster, S. J., Lymphoma Program Investigators at the University of Pennsylvania. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N. Engl. J. Med. 384, 673–674 (2021).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Bruna, R. et al. Prolonged survival in the absence of disease-recurrence in advanced-stage follicular lymphoma following chemo-immunotherapy: 13-year update of the prospective, multicenter randomized GITMO-IIL trial. Haematologica 104, 2241–2248 (2019).
Awasthi, R. et al. Tisagenlecleucel cellular kinetics, dose, and immunogenicity in relation to clinical factors in relapsed/refractory DLBCL. Blood Adv. 4, 560–572 (2020).
Mueller, K. T. et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 130, 2317–2325 (2017).
Jacobson, C. et al. Primary analysis of ZUMA-5: a phase 2 study of axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory indolent non-Hodgkin lymphoma. In 62nd ASH Annual Meeting and Exposition Abstract 700 (2020).
Assouline, S. E. et al. Mosunetuzumab shows promising efficacy in patients with multiply relapsed follicular lymphoma: updated clinical experience from a phase I dose-escalation trial. In 62nd ASH Annual Meeting and Exposition Abstract Abstract 702 (2020).
Hutchings, M. et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J. Clin. Oncol. 39, 1959–1970 (2021).
Schuster, S. J. et al. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood 134, 6 (2019).
Chavey, W. E. 2nd et al. Guideline for the management of heart failure caused by systolic dysfunction: part I. Guideline development, etiology and diagnosis. Am. Fam. Physician 64, 769–774 (2001).
Acknowledgements
The study was sponsored and designed by Novartis Pharmaceuticals Corporation and was approved by the IRB at each participating institution. Data were analyzed and interpreted by the sponsor and the authors. All authors reviewed the manuscript and approved of the final version before submission. We vouch for the data and analysis and for the adherence of the study to the protocol. We thank A. A. Abozeid (former employee of Novartis) for his contributions to the analyses presented herein. Medical writing support was provided by R. Parthasarathy (Healthcare Consultancy Group) and was funded by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Contributions
N.H.F., M.D., S.J.S. and C.T. contributed to the study design. N.H.F., M. Dickinson, M. Dreyling, J.M.-L., A.K., J.B., M.G., L.P., J.C.C., E.B., K.K., H.H., M.J.K., C.A., P.A.R., P.J.H., J.A.P.-S., A.I.C., L.J.N., B.v.T., A.J.M.F., T.T., P.E.M.P., J.P.M., A.L.P., F.O., A.V., P.L.Z., R.M., S.J.S. and C.T. enrolled and treated patients and gathered data. Data were analyzed and interpreted by all authors. All authors participated in the writing and review of this manuscript, and approved the final submitted version.
Corresponding author
Ethics declarations
Competing interests
N.H.F. is an advisor or consultant for Roche/Genentech, TG Therapeutics, Verastem, Bayer, Celgene and Novartis and reports research support from Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, AbbVie and BeiGene. M. Dickinson is an advisor or consultant for Novartis, Bristol-Myers Squibb, Gilead Sciences, Roche and Janssen; has received honoraria from Roche, Amgen, MSD, Janssen, Bristol-Myers Squibb and Novartis; has participated in speaker bureau for Novartis; and reports research support from Novartis, Roche, Takeda, Celgene and MSD. M. Dreyling is an advisor or consultant for AstraZeneca, Bayer/Vital, BeiGene, Celgene/Jazz, Genmab, Gilead Sciences, Incyte, Janssen-Cilag, Novartis and Roche; has participated in speaker bureau for Amgen, AstraZeneca, Bayer Health, Celgene, Gilead Sciences, Janssen-Cilag and Roche Pharma AG; and reports research support from AbbVie, Bayer, Celgene, Janssen-Cilag and Roche Pharma AG and travel support from Celgene, Janssen-Cilag and Roche Pharma AG. J.M.L. is an advisor or consultant for Janssen, Novartis, BMS, Incyte, Astellas and GlaxoSmithKline; reports research support from Bristol-Myers Squibb and Roche; has participated in speaker bureau for Janssen, Novartis, BMS, Incyte, Astellas and GlaxoSmithKline; and owns stock in Altum Sequencing. A.K. is an advisor or consultant for Nordic Nanovector and reports research support from Merck and Nordic Nanovector and travel support from Nordic Nanovector. J.B. is an advisor or consultant for Novartis and has participated in speaker bureau for Novartis. M.G. declares no competing interests. L.P. reports honoraria from Pfizer and Roche and travel support from Novartis. J.C.C. is a consultant for Kite/Gilead, Novartis, Karyopharm Therapeutics, MorphoSys, TeneBio, AbbVie, ADC Therapeutics and Janssen; has participated in speaker bureau for AstraZeneca, BMS, BeiGene and MorphoSys; and reports research support from Merck, AstraZeneca and ADC Therapeutics (Inst.). E.B. is an advisor or consultant for Roche and Incyte and reports research support from Takeda and Amgen, financial support from Roche, Janssen, Celgene, Novartis and Gilead and nonfinancial support from Roche, BeiGene, Celgene and AbbVie. K.K. is an advisor or consultant for AbbVie, AstraZeneca, Celgene, Chugai, Eisai, Janssen and Novartis; has received honoraria from Celgene, Chugai, Janssen, Kyowa Kirin, Merck, Mundi, Novartis, Ono, Sumitomo Dainippon and Takeda; and reports research support from AbbVie, Celgene, Chugai, Eisai, Janssen, Kyowa Kirin, Ono, Novartis and Takeda. H.H. has received honoraria from BMS, Novartis, Chugai and Janssen and reports research support from Astellas. M.J.K. is an advisor or consultant for, and has received honoraria and travel support from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Kite/Gilead, Merck, Miltenyi Biotec, Novartis and Roche; and reports research support from Celgene, Roche and Takeda. C.A. is an advisor or consultant for Gilead Sciences, Kite Pharma, Karyopharm Therapeutics, Atara Biotherapeutics, Incyte, TG Therapeutics and Epizyme; reports research support from Novartis, GlaxoSmithKline, Amgen, Juno Therapeutics, Celgene and Merck; and reports travel support from Gilead Sciences and Kite Pharma. P.A.R. reports grants and personal fees from Kite/Gilead and Celgene/BMS; grants from MorphoSys, Xencor and Calibr; personal fees from Takeda, Verastem, Karyopharm Therapeutics, BeiGene and Bayer; and grants, personal fees and nonfinancial support from Novartis. P.J.H. reports travel support from Celgene, Janssen, La Jolla and Novartis. J.A.P.S. is an advisor or consultant for Novartis, Janssen, Roche, Jazz Pharmaceuticals, Amgen and Gilead Sciences; reports research support from Novartis, Janssen, Pfizer, Roche and Takeda; reports travel support from Roche, Gilead Sciences and Janssen; and reports patents, royalties or other intellectual property from Entourage Bioscience on cannabinoid derivatives. A.I.C. is an advisor or consultant for MorphoSys, Mesoblast and Genentech and has received clinical trial support (Inst.) from Novartis for submitted work. L.J.N. has received honoraria from Bayer, Celgene, Gilead and Novartis and reports research support from Celgene and Genentech. B.v.T. is an advisor or consultant for BMS/Celgene, Novartis, Pentixafarm, Amgen, Pfizer, Takeda, Merck Sharp & Dohme and Gilead Kite; has received honoraria from Novartis, Roche Pharma AG, Takeda and Merck Sharp & Dohme; reports research funding from Novartis (Inst), Merck Sharp & Dohme (Inst) and Takeda (Inst); and reports travel support from AbbVie, AstraZeneca, Kite-Gilead, Merck Sharp & Dohme, Takeda and Novartis. A.J.M.F. declares no competing interests. T.T. is an advisor or consultant for Merck, Novartis and Takeda; has received honoraria from Bristol-Myers Squibb, Fuji Pharma, Kyowa Kirin, Merck, Nippon Shinyaku, Pfizer, Takeda and Teijin Pharma; reports research support from Astellas, Chugai Pharma, Kyowa Kirin, Novartis and Sanofi; reports grants from the Japan Society for the Promotion of Science and Japan Science and Technology Agency; and has received assistance with manuscript preparation for Janssen and Novartis. P.E.M.P. has received grants or contracts from Roche and Gilead; has received honoraria from AbbVie, Gilead, Janssen and Novartis; has received travel support from AbbVie and Janssen; and has participated on a data safety monitoring board or advisory board for AbbVie and Novartis. J.P.M. is an advisor or consultant for Juno; has received honoraria from AlloVir, Juno and Kite; reports research support from AlloVir, Astellas, Bellicum, Fresenius Biotech, Gamida Cell, Juno, Kite, Novartis and Pluristem; has participated in speaker bureau for Kite; and has received assistance with manuscript preparation for ArticulateScience LLC. A.L.P. is an advisor or consultant for, and reports honoraria from, Novartis, BMS, Pfizer, Roche, Takeda, Celgene-BMS and Incyte and travel support from Celgene-BMS and Pfizer. F.O. declares no competing interests. A.V. is advisor or consultant for Novartis, Kite/Gilead, BMS, Amgen and Roche and reports personal fees and nonfinancial support from Roche, BMS, Novartis, Amgen, Takeda, AstraZeneca and Kite/Gilead. P.L.Z. is an advisor or consultant for Merck Sharp & Dohme, Bristol-Myers Squibb, Celgene, Gilead, Infinity, Janssen, Pfizer, Roche and Takeda. R.M. is an advisor or consultant for Roche and reports travel support from Amgen, Novartis and Gilead Sciences. A.Z., R.A., A.M. and O.A. are employees of Novartis. S.J.S. is an advisor or consultant for Acerta, AlloGene, AstraZeneca, BeiGene, Celgene/Juno, Genentech/Roche, LoxoOncology, Novartis and Tessa Therapeutics; has received honoraria from Acerta, AlloGene, AstraZeneca, BeiGene, Celgene, Genentech/Roche, LoxoOncology, Novartis, Nordic Nanovector, Pfizer and Tessa Therapeutics; has participated in a steering committee for AbbVie, Celgene, Novartis, Juno, Nordic Nanovector and Pfizer; reports research support from AbbVie, Acerta, Celgene/Juno, DTRM Bio, Genentech, Incyte, Merck, Novartis, Portola and TG Therapeutics; and holds a patent with Novartis. C.T. has received honoraria from Celgene, AbbVie, Bayer, Janssen, Roche, Incyte, Novartis and Gilead and reports research funding from Roche and travel support from Roche, Janssen-Cilag, Kite/Gilead and Novartis.
Peer review information
Nature Medicine thanks Jeremy Abramson, Ji-Hyun Lee, Jordan Gauthier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Saheli Sadanand was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cytokine release syndrome within 8 weeks of tisagenlecleucel infusion.
CRS=cytokine release syndrome; ICU=intensive care unit; IQR=interquartile range. Column titles are bolded for clarity.
Extended Data Fig. 2 Resolution of hematopoietic cytopenias post-tisagenlecleucel infusion.
aBased on laboratory results regardless of blood transfusion. bResolution of cytopenia is defined as achieving lab results of grade ≤2. Percent resolved probability is among subjects with cytopenia at week 4, obtained from the Kaplan-Meier survival estimates. cNumber of subjects with last value on or prior to week 4, indicating grade 3 or 4 cytopenia. dWeek 4 is defined as day 35. WBC=white blood cells. Column titles are bolded for clarity.
Extended Data Fig. 3 Neurological events within 8 weeks of tisagenlecleucel infusion.
aG4 ICANS: Onset D10, recovered – Related to tisagenlecleucel. Patient presenting with tremors, then seizures, with concomitant HHV6 positivity on CSF. The event fully recovered after high-dose MPD and GCV. CSF=cerebrospinal fluid; GCV=ganciclovir; ICANS=immune effector cell-associated neurotoxicity syndrome; HHV6, Human Herpesvirus 6; MPD=methylprednisolone. Column titles are bolded for clarity.
Extended Data Fig. 4 Summary of cellular kinetic parameters for tisagenlecleucel.
AUC0-28d=mean area under the concentration-time curve from day 0 to day 28; AUC0-84d=mean area under the concentration-time curve from day 0 to day 84; Cmax=geometric mean maximum expansion; CR=complete response; PD=progressive disease; PR=partial response; SD=stable disease; Tmax=time to maximal expansion; Tlast=duration CAR-T cells are present in peripheral blood and tissues. Column titles are bolded for clarity.
Supplementary information
Supplementary Information
Redacted study protocol.
Supplementary Data
Redacted CSR and SAP.
Rights and permissions
About this article
Cite this article
Fowler, N.H., Dickinson, M., Dreyling, M. et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med 28, 325–332 (2022). https://doi.org/10.1038/s41591-021-01622-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01622-0
This article is cited by
-
Toxicities, intensive care management, and outcome of chimeric antigen receptor T cells in adults: an update
Critical Care (2024)
-
Optimizing Real-World Outcomes in High-Risk Relapsed/Refractory (r/r) FL with CAR-T Cell Therapy: A Vodcast and Case Example
Oncology and Therapy (2024)
-
Budget Impact of Introducing Fixed-Duration Mosunetuzumab for the Treatment of Relapsed or Refractory Follicular Lymphoma After Two or More Lines of Systemic Therapy in the USA
PharmacoEconomics (2024)
-
Phase II study of novel orally PI3Kα/δ inhibitor TQ-B3525 in relapsed and/or refractory follicular lymphoma
Signal Transduction and Targeted Therapy (2024)
-
A multi-cohort phase 1b trial of rituximab in combination with immunotherapy doublets in relapsed/refractory follicular lymphoma
Annals of Hematology (2024)