Abstract
Imaging with positron emission tomography (PET)/computed tomography (CT) is crucial in the management of cancer because of its value in tumor staging, response assessment, restaging, prognosis and treatment responsiveness prediction. In the last years, interest has grown in texture analysis which provides an “in-vivo” lesion characterization, and predictive information in several malignances including NSCLC; however several drawbacks and limitations affect these studies, especially because of lack of standardization in features calculation, definitions and methodology reporting. The present paper provides a comprehensive review of literature describing the state-of-the-art of FDG-PET/CT texture analysis in NSCLC, suggesting a proposal for harmonization of methodology.
Similar content being viewed by others
Introduction
Positron emission tomography (PET)/computed tomography (CT) using the radiopharmaceutical 18F-fluoro-deoxy-glucose (FDG) has a paramount role in the management of cancer patients owing to its value in tumor staging, response assessment, and restaging as well as in prognosis and prediction of treatment response. The standardized uptake value (SUV) obtained from FDG-PET scans is the most widely used parameter for lesion characterization and it has been shown to have a prognostic value1. More recently, volumetric parameters, including metabolic tumor volume (MTV) and total lesion glycolysis (TLG), have also been proposed for assessment of prognosis2,3,4,5. Moreover, in recent years there has been emerging evidence that the heterogeneity of density values on CT and of FDG uptake within the primary tumor can permit in vivo lesion characterization and provide predictive information in malignances, including non-small cell lung cancer (NSCLC)6,7,8,9,10.
The term “heterogeneity” conveys different meanings depending on the imaging modality: in FDG-PET it refers to the variability in the distribution of radiopharmaceutical uptake, while in CT it refers to the variability in tissue density. Lesion “heterogeneity” can be described by a multitude of mathematical methods that, taken together, constitute the “texture analysis” which provides numerous quantitative and semiquantitative indices, termed “features”11,12,13. This approach as a whole is named “radiomics”.
Textural features seem to perform better than the conventional uptake parameters used for image interpretation in clinical routine, such as SUV measurements (e.g., SUVmax, SUVmean), which are subject to several limitations14. Numerous studies have explored the additional information that can be extracted by texture analysis, with the aim of characterizing tumor lesions. However, these investigations have had multiple drawbacks and limitations, especially with respect to lack of standardization in feature calculation, definitions, and reporting methodology15.
Texture analysis has the potential to impact on patient management if its ability to characterize lesions in vivo and to provide predictive information is demonstrated in prospective studies. As lung cancer is the fourth most frequently diagnosed malignancy in Europe and the the leading cause of cancer mortality (http://eco.iarc.fr/eucan), texture analysis in such patients, when validated, will strongly impact on patient management and healthcare systems. The present article provides a comprehensive review of the literature describing the state-of-the-art in FDG-PET/CT texture analysis methods in lung cancer. It also reports on the ability of textural features to identify tumor phenotype and to provide additional predictive and prognostic information in patients with NSCLC. Moreover, a comprehensive review of calculation methods, feature names, and definitions is performed, and a scheme for harmonization of methodology and reporting of results is proposed.
Methods
From the PubMed/MEDLINE database a search algorithm based on a combination of the following terms was used: (a) “texture” or “textural” or “radiomics” or “heterogeneity” or “heterogeneous” or “features” or “histogram” and (b) “lung cancer” or “NSCLC” and (c) “PET” or “PET/CT”. No start date limit was used and the search extended to 23 April 2016. To expand our search, references of the retrieved articles were also screened. Two authors independently searched articles and performed an initial screening of identified titles and abstracts. All studies or subsets in studies investigating the role of PET or PET/CT radiomics in patients with suspected/definite NSCLC were considered eligible. The exclusion criteria were: (a) articles not within the field of interest; (b) review articles, editorials or letters, comments, and conference proceedings; (c) articles not in the English language; (d) case reports or small case series (<10 patients); and (e) in vitro or animal studies.
Among the 294 studies identified by reviewing titles and abstracts, 247 were excluded by applying the criteria mentioned above. One paper not retrievable in the full-text version was excluded. Forty-six articles, retrieved in full-text version, were assessed for eligibility. Nine articles were excluded after reading the full text version. One further article was identified after screening of the references. Overall, 38 articles were selected and used for the qualitative synthesis (Fig. 1). Considering the aim of this review (i.e. texture analysis in NSCLC) which takes into account a variety of heterogeneous papers in terms of aim(s), methods, and results; a systematic review according to the PRISMA algorithm16 was considered not feasible. Selected papers were grouped into two different sets based on their aims: technical/methodological and clinical studies. The technical/methodological group included 16 papers (568 patients) that tested specific algorithms, different approaches for segmentation and tumor volume delineation, modalities of image acquisition, attenuation correction, or reconstruction. The clinical set comprised 22 papers (2306 patients), and we separately reviewed the results in respect of the diagnostic and the prognostic or predictive role of the textural features in NSCLC. Although prognosis and treatment prediction should be reported separately, in order to avoid an overlap of contents we treated these topics in the same section since many of the analyzed studies have evaluated them simultaneously. In approaching this review, the main difficulties were related to the differences in textural feature nomenclature and to the comparison of results regarding features obtained using different approaches. Therefore, we started with a reclassification of each feature (Fig. 2). For each feature we specified the order (i.e., first, second, or superior), the matrix (e.g., histogram, gray-level co-occurrence), the definition, and/or the formula, when available, as reported in the Supplementary material. Hereafter the features that are denominated in the identical way but can be derived using different approaches (e.g., histogram, gray-level co-occurrence matrix) are reported by adding the matrix from which they have been derived in subscript.
Results
Nomenclature and methods in texture analysis
Texture analysis refers to a variety of mathematical methods that may be applied to describe the relationships between the intensity of pixels or voxels and their position within an image. An advantage of measuring textural parameters is that it is a post-processing technique that can be applied to data acquired during routine clinical imaging protocols, thereby maximizing the information that can be derived from standard clinical images14. Distinct approaches (statistics based17, 18, model-based19,20,21, transform-based22,23,24, and structural25) may be used to analyze functional imaging information, resulting in numerous radiomics features, such as descriptors of the image intensity histogram, “shape and size” features, descriptors of the relationships between image voxels (e.g., GLCM-, and NGTDM-derived features), textures extracted from wavelet and Laplacian of Gaussian filtered images, and fractal features26, as shown in Fig. 2.
The first approach consists in the summarizing of 3D functional imaging data into a single curve – histogram – representing the voxel intensity values contained within the volume of interest (VOI), allowing for a simplified interpretation. Intensity-volume histograms (IVH) or cumulative SUV-volume histograms (CSH) have been proposed by El Naqa et al.27 as a novel way to characterize heterogeneity in tumor tracer uptake. These histograms are similar to dose-volume histograms frequently used in radiotherapy28. A set of metrics are derived from IVH representations that reflect the voxel value frequency distribution27: Ix (minimum intensity to x% highest intensity volume); Vx (percentage volume having at least x% intensity value); and descriptive statistics (mean, minimum, maximum, standard deviation, etc.)29. In CSH the percent volume of a tumor (derived from CT or from PET-based (semi-)automatic tumor delineation methods30) with an SUV above a certain threshold is plotted against that threshold value, which is varied from 0 to 100% of SUVmax. The area under the CSH (AUC-CSH) may be a quantitative index of tracer uptake heterogeneity and/or heterogeneous tumor response31. Any method to characterize heterogeneity, however, will treat both partial volume effect and noise as heterogeneity. Therefore partial volume correction (PVC) and image denoising should be applied prior to calculating AUC-CSH32. Despite this consideration, PVC has not been routinely applied. To overcome this potential limitation on the quantitative measurements, most studies have considered relatively large lesions (generally volumes >3–5 cm3), assuming that PET cannot characterize heterogeneity in smaller volumes because of its limited spatial resolution.
IVH and other first-order approaches are limited by their spatial insensitivity. To overcome this drawback, textural features and “shape and size” attributes may be extracted that contain embedded spatial and topological information. In fact, second-order and high-order statistics (i.e., based on gray-level matrix, nearest neighbor spatial dependence matrices, etc.) provide information from the spatial relationship of image voxels33. The gray-level co-occurrence matrix features may be used to represent texture information because of its relatively simple and intuitive structure. Surface plots of the co-occurrence matrix give a pictorial representation of the spatial-intensity distribution, which is typically masked by first-order histogram analyses. Several other matrices are also used, including the neighborhood gray-tone difference matrix, which provides information regarding how each voxel value differs from the neighboring voxel values; the gray-level run length matrix, which stores the number of voxels with identical values in each direction; and the gray-level size zone matrix, which stores the size of the 3D region that includes a given voxel value27, 29, 33,34,35,36,37. Therefore, a multitude of metrics may be derived from the gray-level matrices to characterize the structure of interest27. These metrics are independent of tumor position, orientation, size, and brightness and take into account the local intensity-spatial distribution33, 38, 39. Hence, the combination of these features can provide an intensity-spatially dependent map of the tumor metabolic uptake that can potentially be used as a signature to characterize the tumor phenotype and response to treatment.
Texture feature extraction requires the voxel intensity values within the VOI to be discretized. This discretization step not only reduces image noise, but also normalizes intensities across all patients, allowing for a direct comparison of all calculated textural features between patients. Shape and size features are calculated, describing the 3D shape and size of the lesions40. However, it should be considered that volumetric indexes (e.g., sphericity) may also be extracted from the IVH40,41,42, allowing for a simplified interpretation27.
The majority of texture features that have been used in PET medical imaging to date fall into one of the following three categories: (a) first-order features derived from statistical moments of the image intensity histogram, (b) second-order features derived from the GLCM, and (c) higher order features derived from analysis of the NGTDM, NGLDM, or GLSZM43. Despite the difficulties in generalization due to the variability in textural PET features among studies, related to the methodology used, we can summarize that IVH features tend to depend on the tumor delineation method44,45,46 and that features derived from GLSZM have been reported to be the most susceptible to variability40, 46, 47, while the GLCM-derived features tend to be the most robust. In particular, “entropyHist” and “entropyGLCM” have been reported to be features less dependent on the tumor segmentation method46, reconstruction settings, iteration numbers, and voxel size47, and type of acquisition (3D versus 4D)42, 48.
Texture analysis and technical/methodological investigations
The accuracy and precision of texture analysis derived from PET images depends significantly on scanning protocols. Factors such as image acquisition, reconstruction, and inherent image quality parameters (noise, motion artifacts, and slice thickness) may be important. It is to be expected that all texture analysis methods are influenced to some extent by these factors and the sensitivity of various textural features may be based on different image models. Further aspects that require careful assessment are the methods used for region of interest (ROI) definition on PET images and the intraobserver and interobserver variations14. Figure 3 shows examples of different methods used for ROI definition. All of these aspects have been evaluated in the determination of PET features in NSCLC patients (Table 1).
Example of tumor contouring using in (a) a threshold method at 50% of SUVmax and (b) a method based on an absolute SUV cut-off of 2.5. The ROI identified by using the absolute SUV cut-off of 2.5 is greater than that identified by the threshold method, as shown by axial (top), sagittal (right), and coronal (left) images (same slices).
Hatt et al.44 evaluated the impact of five different methods of segmentation on anatomic tumor volume, MTV, and heterogeneity (“coefficient of variation” histogram-based) in a small group of NSCLC patients. They found that all delineation methods except the manual one resulted in an underestimation of MTV, and that larger lesions were more heterogeneous.
Similar results were obtained in a larger population (n = 50) with NSCLC. Tumor volume was observed to be significantly diverse using different approaches (manual or automatic) on CT and on fused PET/CT images (volumes delineated on CT were larger than those defined on PET images). Intratumor heterogeneity, defined by visual scoring, “coefficient of variation”, or “entropyGLCM” (gray-level co-occurrence matrix – GLCM) significantly correlated with differences in the target volume [tumors with a high heterogeneity showed a larger gross tumor volume (GTV)], suggesting that caution should be exercised when applying relatively simple threshold-based segmentation to define the target volume for tumors with high heterogeneity45.
Hofheinz et al.49 developed and tested a voxel-specific threshold algorithm as a delineation method for heterogeneous tumors. This method, which can be considered as an extension of an adaptive threshold method, proved able to reproduce the true tumor boundaries accurately, without being influenced by the heterogeneity (“coefficient of variation”).
Cui et al.50 developed an automatic algorithm that used the PET SUV volume and the CT volume to localize and segment tumor lesions. This algorithm outperformed other (semi-)automatic methods in terms of overlapping measure, and they found that the feature “contrastNGTDM” (NGTDM: neighbor gray-tone difference matrix based) was valuable in automatic tumor localization. The same group developed and tested a “topo-poly” algorithm (which incorporated an intensity graph and a topology graph) in two groups of patients defined as having ‘isolated’ (i.e., lung tumor located in the lung parenchyma and away from associated structures/tissues in the thorax) or ‘complex’ (i.e., tumor abutted/involving a variety of adjacent structures, where the tumor margins were indistinct and/or had heterogeneous regions of FDG uptake) disease. This method provided better anatomic and functional boundary delineations for both small and large tumors and for ‘complex’ cases. Again, “contrastNGTDM” was valuable in automatic tumor localization51.
Leijenaar et al.40 tested more than 100 PET features (first-order statistics and intensity volume histogram – FOS/IVH, “shape and size” features – SS, GLCM, gray-level run-length matrix – GLRLM, and gray-level size-zone matrix – GLSZM) to evaluate their test–retest reliability and interobserver stability among different tumor delineation methods in 34 NSCLC patients. Considering all features, a good overall similarity in feature stability was observed, based on rankings in terms of test–retest and interobserver intra-class correlation coefficient (ICC, p ≪ 0.001). Comparing stability rankings per feature group, a high similarity was found for both the first-order statistics (p ≪ 0.001) and other textural features (p ≪ 0.001). Features based on GLSZM had the overall lowest ranks, indicating that these features have the highest variability. For the IVH features the observed similarity was more moderate (p ≪ 0.001). Comparison of the rankings for the geometric features proved non-significant (p = 0.086). Overall, more stable features on repeated PET scans were also more robust against interobserver variability. In a similar number of patients, the same group evaluated prospectively different discretization methods (fixed bin size versus fixed number of bins) for textural feature extraction in the context of treatment response assessment. Textural feature values were shown to depend on the intensity resolution used for SUV discretization. Discretizing SUVs using a fixed number of bins was found to be less appropriate for inter- and intrapatient comparison of textural feature values in a clinical setting. Additionally, results obtained for the features could not be directly compared when different intensity resolutions were used, suggesting that their interpretation (e.g., prognostic or predictive value) depended on the intensity resolution. It is noteworthy that the “correlationGLCM” was the only feature observed to have highly similar patient rankings over the course of treatment, regardless of the discretization method or discretization value used52.
Orlhac et al.46 investigated a consistent number of texture indices on a variety of tumors (including 24 NSCLC) to gain a better insight into how they relate to one another and to conventional indices such as SUV, MTV, and TLG and to determine the extent of their robustness with respect to the gray-level resampling scheme and formula and to the tumor delineation method. All histogram indices strongly depended on the tumor delineation method. Similarly, “contrastNGTDM”, “busyness” (NGTDM-based), “low gray-level run emphasis”, “short-run low gray-level emphasis”, “long-run low gray-level emphasis” (GLRLM-based), “low gray-level zone emphasis”, and “short-zone low gray-level emphasis” (GLSZM-based) were highly sensitive to the segmentation method, while “homogeneityGLCM” and “entropyGLCM” were found to be robust with respect to tumor segmentation. The same group investigated, in 48 treatment-naïve NSCLC patients, the effect of the resampling approach on the ability of textural features to reflect tissue-specific patterns of metabolic activity. An adaptive threshold method was used to delineate tumors. The relative resampling approach (RR) was compared with the absolute resampling (AR) approach. Seven features (from GLCM, GLRLM, and GLSZM) were calculated and correlated with tissue types and cancer subtypes. AR-based “entropyGLCM” could differentiate between tumor and healthy tissue (p < 0.0001). Using the AR method, tumor tissue exhibited higher “high gray-level zone emphasis” than healthy tissue, while tumors had lower “homogeneityGLCM” and “low gray-level zone emphasis”. AR-based textural features differed adenocarcinoma (Adk) and squamocellular carcinoma (Sqc) (p ≤ 0.05)53.
Yan et al.47 tested the variability of more than 60 PET-textural features using different reconstruction settings, different iteration numbers, and different voxel size in 17 NSCLC patients. “Skewness” (IVH-based), “cluster shadeGLCM”, and “zone percentage” (GLSZM-based) were the least robust with respect to reconstruction algorithms using default settings and were the most sensitive to iteration number. Among all the features evaluated, “entropyHist”, “difference entropy”, “inverse difference normalized”, “inverse difference moment normalized”, “low gray-level run emphasis”, “high gray-level run emphasis”, and “low gray-level zone emphasis” proved to be the most robust.
Recently, repeatability of more than 100 radiomics features using different reconstruction settings, first using the point spread function and secondly complying with the European Association of Nuclear Medicine (EANM) guidelines for tumor PET imaging54, and using different delineation methods, first on PET and then on CT images, was evaluated in 11 NSCLC patients. The best performance was seen using CT-based delineation (32%), followed by EANM-compliant reconstruction (17%), PET-based delineation (17%), and point spread function-based reconstruction (10%). The majority of PET features (98%) had a repeatability comparable to that reported for simple SUV measures (e.g., SUVmax) in the literature. Sixty-three features showed a very high ICC (≥0.90) independent of delineation or reconstruction. The performance of radiomics features depended more on the delineation method than on the applied reconstruction algorithm (changes in 25 and 3 features, respectively). CT-based delineation showed favorable repeatabilities and ICCs for most radiomics features, an exception being shape-based features, for which PET-based delineation performed better41.
Compared with static images, parametric images don’t provide significant complementary information concerning standard parameters (SUVmax, SUVmean, and metabolically active tumor volume - MATV) and heterogeneity quantification (histogram-based)55. Differences in quantitative analysis using three-dimensional (3D) versus respiratory-gated (4D) acquisition have been reported. According to Oliver et al.42, the features with the least variability were “sphericity”, “spherical disproportion”, “entropyHist”, “entropyGLCM”, “sum entropy”, “information measure of correlation 2”, “short run emphasis”, “long run emphasis”, and “run percentage”, while the features with the largest differences (>50%) were “kurtosis”, “low gray-level run emphasis”, “short run low gray-level emphasis”, and “long run low gray-level emphasis”.
Yip et al.56 found significant differences in “maximal correlation coefficient”, “long run low gray-level emphasis”, “coarseness”, and “busyness” (NGTDM-based) between 3D and 4D PET imaging. When measuring tumor heterogeneity characteristics, reduced motion blurring by 4D PET acquisition was found to offer significantly better spatial resolution of textural features. 3D PET textures may lead to inaccurate prediction of treatment outcome, hindering optimal management of lung cancer patients. 4D PET textures may have a better prognostic value as they are less susceptible to tumor motion42, 56. Different results have been reported by Cheng et al.48, who compared the attenuation correction of PET images with helical CT (PET/HCT) and respiration-averaged CT (PET/ACT) in 56 NSCLC patients. PET/ACT yielded significantly higher SUVmax, SUVmean, and TLG while significant differences between PET/HCT and PET/ACT were not observed with regard to other features, including “entropyHist”, “entropyGLCM”, “dissimilarity”, “homogeneityGLCM”, and “uniformity” (GLCM-based), “gray-level non-uniformity”, “zone-size non-uniformity”, and “high gray-level large zone emphasis” (GLSZM-based), and “coarseness”, “busyness”, “contrastNGTDM”, and “complexity” (NGTDM-based).
Textural features have also been used to develop a computer-based algorithm which supported a vector machine; combined image parameters, derived from CT, PET, and PET/CT images, were found to improve diagnosis of mediastinal lymph node metastases by PET/CT57.
Texture analysis and clinical applications
Diagnosis
Imaging texture analysis has been evaluated in order to determine which type and level of tissue heterogeneity can be captured and quantified through PET and to bridge the gap between in vivo and ex vivo tumor characterization58. Histologic characteristics and PET features have been compared to identify whether texture analysis can help in differentiating between benign and malignant lesions or in classifying NSCLC subtypes (Table 2).
As mentioned above, Orlach et al.53 compared the relative with the absolute resampling approach (RR and AR, respectively) and calculated the correlations of seven features with tissue types (tumor versus healthy tissue) and cancer subtypes (Adk versus Sqc). RR-based “entropyGLCM” didn’t distinguish between tumor and healthy tissue (p = 0.7621) whereas the same index computed with the AR method was able to differentiate between these tissue types (p < 0.0001). Using the AR method, tumor tissue exhibited higher “high gray-level zone emphasis” than healthy tissue, while tumors had lower “homogeneityGLCM” and “low gray-level zone emphasis”. Comparing textural indices in Adk versus Sqc, all RR-based textural features were not significant (p > 0.07), in contrast to the AR-based textural features (p ≤ 0.05). According to these results, features computed using an AR method vary as a function of the tissue type and cancer subtype and might be useful for tumor characterization.
Miwa et al.37 evaluated whether morphological complexity (“morphological fractal dimension” derived from CT) and intratumoral heterogeneity (“density fractal dimension” derived from PET) assessed by fractal analysis improved the differential diagnosis between benign and malignant lung nodules in 54 patients with suspected NSCLC. Both fractal dimensions assessed by PET and CT were lower in malignant than in benign nodules (p < 0.05). SUVmax was higher in malignant than in benign nodules (p < 0.05). Tumor size significantly correlated with SUVmax (p < 0.0001), but not with either “morphological fractal dimension” (p = 0.61) or “density fractal dimension” (p = 0.09). The diagnostic accuracy of “density fractal dimension” tended to be higher than SUVmax (78% versus 68%, respectively) and was better than that for “morphological fractal dimension” (65%).
Heterogeneity has also been evaluated to determine whether it can help in differentiating between metastatic and inflammatory lymph nodes in lung Adk, as assessed by visual analysis, and other standard parameters of PET and CT (size and Hounsfield units). In this study, heterogeneity was assesed as “coefficient of variation” of lymph nodes in 44 patients (with a total of 94 biopsy-proven lymph nodes). Visual assessment for malignancy had high sensitivity (81%) but a relatively low specificity (67%), with an accuracy of 75%. The diagnostic performance of PET/CT using the cut-offs commonly employed for standard PET and CT parameters (SUVmax = 2.5, size of lymph nodes = 1 cm, and Hounsfield units = 120) was not satisfactory (accuracy of 56%, 60%, and 68%, respectively). Using an optimal cut-off determined by this study (SUVmax = 5.96, size of lymph nodes = 1.5 cm, and Hounsfield units = 136), the accuracy increased for SUVmax and size but not for Hounsfield units (81%, 84%, and 65%, respectively). Heterogeneity measured as “coefficient of variation” (using a cut-off = 0.2) yielded good sensitivity, specificity, and accuracy (88%, 76%, and 82%, respectively). The accuracy of “coefficient of variation” was slightly higher than that of SUVmax and size when using optimal cut-offs, but significantly higher than that of visual assessment and Hounsfield units. “Coefficient of variation”, SUVmax and size were significantly higher in metastatic lymph nodes than in benign ones (p < 0.0001), while the Hounsfield unit value was significantly lower in metastatic than in benign lymph nodes (p = 0.0249). Univariate analysis showed that all parameters except visual assessment were significant predictors, while using multivariate logistic regression only “coefficient of variation” and size proved statistically significant (p = 0.032 and 0.023, respectively)59.
Yip et al.56, in order to evaluate whether texture features may be affected differently in Adk (21 lesions) versus Sqc (13 lesions) by motion, calculated the relative difference in each texture between 3D and 4D PET. The relative difference in each texture between 3D and 4D PET was not found to be significantly different between histologies (p = 0.26).
Ha et al.60 analyzed differences in 24 textural features between Adk and Sqc (17 and 13 patients, respectively). The majority of texture parameters that showed a significant difference between Adk and Sqc were derived from GLCM (93%). SUVmax showed the most significant association with tumor pathology (p = 0.001). Upon autoclustering by linear discriminant analysis with those texture parameters that showed a significant difference between tumor subtypes (n = 15), the classification accuracy was found to be 83% (25/30 lesions were correctly clustered to their own tumor subtype). When analyzing with all parameters (n = 24), linear discriminant analysis clustered the lesions accurately according to their pathology, i.e., Adk versus Sqc, with a classification accuracy of 100% (linear separability of this autoclustering = 0.90).
Similarly, Kim et al.61 found that SUVmax, MTV, TLG, and heterogeneity (defined as the derivative of the volume-threshold function from 20 to 80%), were significantly higher in Sqc than in Adk.
van Gómez López et al.62 evaluated the correlation between conventional metabolic parameters (SUVmax, SUVmean, MTV, and TLG) and heterogeneity (“energyGLCM”, “contrastGLCM”, “correlation”, “entropyGLCM”, and “homogeneityGLCM”), histology, tumor size, and AJCC stage in 38 NSCLC patients (24 Sqc and 14 Adk). There was a positive relationship for all metabolic parameters with “entropyGLCM”, “correlation”, and “homogeneityGLCM” and a negative relationship with “energyGLCM” and “contrastGLCM”. No statistically significant differences were found between the mean values of tumor size, AJCC stage, and standard metabolic parameters in Adk versus Sqc tumors. Concerning textural features, “energyGLCM” was lower in Adk than in Sqc (p = 0.027) while “homogeneityGLCM” was higher in Adk than in Sqc (p = 0.047). Tumor size was correlated with “energyGLCM”, “contrastGLCM”, “correlation”, “entropyGLCM”, “MTV”, and “TLG” (p < 0.01). A statistical correlation between the pT and “energyGLCM”, “contrastGLCM”, “entropyGLCM”, and “MTV” (p ≤ 0.05) was found, but not between remaining AJCC subgroups and the other textural or metabolic parameters.
Prognosis and treatment response prediction
The Warburg effect, first described over 80 years ago, postulates that tumors undergo glycolysis preferentially despite adequate intracellular oxygen tension63, 64. While Warburg believed this to be a consequence of mitochondrial dysfunction, tumor glycolysis can proceed with functional cellular mitochondria and may be an adaptive response for tumor survival65,66,67. Furthermore, studies have recently linked glycolysis in cancer to more widespread deregulation of cell bioenergetics68,69,70, suggesting that FDG uptake may be a surrogate for more than glycolysis alone and perhaps a lens through which one can view global tumor bioenergetics71. Therefore, texture features have been introduced as imaging biomarkers on the assumption that they are an index of the degree of tumor heterogeneity, and that biologic tumor heterogeneity is associated with poor prognosis in cancer patients and can contribute to treatment failure and drug resistance72. The prognostic value of texture analysis has been evaluated in different NSCLC clinical settings (Table 2).
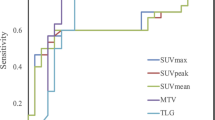
Nair et al.71 evaluated a possible association between textural features, gene expression signatures, and survival in a computational study (172 NSCLC patients). Fourteen PET features were extracted within the study cohort (n = 25). Individual genes associated with PET features in the study cohort were directly analyzed in the external cohort (n = 63) for their association with clinical outcomes. Lastly, PET features associated with prognostic gene signatures from the external cohort were tested in a validation cohort (n = 84). Four genes (LY6E, RNF149, MCM6, FAP) associated with textural features were found also to be associated with survival. Histogram-based and “shape and size” features together provided a more accurate prognostic model than each feature alone, suggesting that leveraging tumor genomics with an expanded collection of PET features may enhance understanding of the value of FDG uptake as an imaging biomarker beyond its association with glycolysis.
Win et al.13 compared the prognostic value of texture analysis with tumor staging and other imaging prognostic factors (i.e., metabolism assessed by PET/CT and permeability assessed by dynamic contrast-enhanced CT) in 122 NSCLC patients treated with curative or palliative approach. Tumor heterogeneity (“entropyHist”) was calculated from both attenuation-corrected CT images and SUV images without image filtration. “EntropyHist” (derived from both CT and PET images), permeability, and stage were found to be survival predictors at univariate analysis (p ≤ 0.003), in contrast to SUVmax (p = 0.948). At multivariate analysis, “entropyHist” derived from CT (p = 0.021), stage (p = 0.001), and permeability (p < 0.001) were identified as independent survival predictors, irrespective of the treatment objective (curative or palliative). In the study by Cheng et al., “EntropyHist”, “entropyGLCM”, and “coarseness”, derived from both PET/HCT and PET/ACT, were able to predict disease-specific survival at univariate (p ≤ 0.01) and multivariate analysis (p < 0.05) in stage I–III NSCLC patients48.
Similarly, the “shape and size” features “asphericity” (p < 0.001) and “solidity” (p = 0.05), as well as “primary surgical treatment” (p = 0.05), were found to be significant independent predictors of progression-free survival in 60 NSCLC patients treated with different approaches. Concerning overall survival, only “asphericity” and “primary surgical treatment” (p = 0.02 and = 0.01, respectively) proved to be independent predictors, and none of the other PET parameters (including SUVmax, TLG, and MTV) showed a significant predictive value in this series of patients73.
The GLCM- and GLSZM-derived features “entropyGLCM”, “homogeneityGLCM”, “dissimilarity”, “size-zone variability”, and “zone percentage”, but not “high intensity emphasis” (GLSZM-based), have also been reported to be independent prognostic factors with respect to stage (although not independently of each other) in patients treated with different approaches. Nonetheless, the addition of risk factors allowed a better differentiation of patient outcome. High SUV, large metabolic volumes, and high heterogeneity were associated with a poorer overall survival and recurrence-free survival35, suggesting that heterogeneity quantification and volume (i.e., MTV) may provide valuable complementary information with respect to prognosis, although the complementary information increases substantially with larger volumes74.
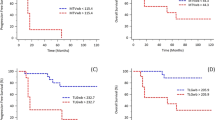
Heterogeneity of primary tumor (evaluated by the area under the curve of cumulative SUV histograms: AUC-CSH) was observed to be an independent predictor of recurrence in pathologically N0 Adk but not in Sqc (p = 0.03 and 0.13, respectively) after curative surgical resection61 as well as a predictor of disease progression after concurrent chemoradiotherapy in patients with inoperable stage III NSCLC75.
Interestingly, “Sum Average” (GLCM-based) was strongly associated with overall survival in a multi-institutional dataset of locally advanced NSCLC patients with large tumors who were treated with definitive chemoradiotherapy, suggesting its robustness as a prognostic factor76.
NGTDM-derived features (“coarseness”, “contrastNGTDM”, and “busyness”) have also been reported to be associated with response to chemoradiotherapy and prognosis77 in locally advanced NSCLC. The same group, testing FOS and high-order features as predictors of response or survival in patients treated with erlotinib, found that response to erlotinib was associated with reduced heterogeneity and that the percentage of changes in “entropyHist” (between baseline and 6-week PET/CT) was independently associated with overall survival and treatment response78.
Similarly, PET features have been reported to be able to predict outcome and/or treatment response in NSCLC patients treated with definitive radiotherapy29, 36, 79, 80. In this specific clinical setting, “entropyGLCM” has been reported to be an independent predictor of disease-specific survival (p = 0.016)79, while “dissimilarity” has been found to be associated with both disease-specific survival (p = 0.037) and disease-free survival (p < 0.01)36.
Initial attempts have been made to determine whether quantitative imaging features from pretreatment PET can enhance overall survival risk stratification beyond what can be achieved with conventional prognostic factors in NSCLC. In patients with stage III NSCLC, linear predictors of overall survival generated with both quantitative imaging features (histogram-derived, GLCM-derived, and “shape and size” features) and conventional prognostic factors (age, sex, histologic findings and stage, Karnofsky performance status, smoking status and estimated pack-years, treatment type) have demonstrated improved risk stratification compared with those generated with conventional prognostic factors alone in terms of log-rank statistics (p = 0.18 versus = 0.0001, respectively)80. The use of quantitative imaging features selected during cross-validation improved the model using conventional prognostic factors alone (p = 0.007). Disease “solidity” and primary tumor “energyGLCM” were found to be selected in all folds of cross-validation81. Additionally, these features were found to be capable of isolating subgroups of patients who received a benefit or detriment from dose escalation82.
Similarly, PET “entropyGLCM” and CT “zone percentage” have been found to have the highest complementary values with clinical stage and functional volume in stage I–III NSCLC83. Desseroit et al.83 provided a nomogram able to improve stratification amongst patients with stage II and III disease, allowing identification of those with the poorest prognosis (clinical stage III, large MTV, high PET heterogeneity, and low CT heterogeneity).
In early-stage NSCLC the optimal prognostic model for prediction of distant metastases in patients treated with stereotactic ablative radiation therapy included two image features that allowed quantification of intratumor heterogeneity and SUVpeak. A significant improvement (p = 0.0001) in predicting freedom from distant metastasis was seen when histologic information was added compared with a prognostic model based solely on imaging features84.
Discussion
Texture features are of growing interest for tumor characterization in imaging. Nevertheless, on the basis of results published to date on FDG PET, it is unclear which indices should be used, what they represent, and how they are related to conventional parameters such as SUVs, MTV, and TLG46. We summarize the results of the available studies within Table 3. PET features differed significantly in malignant and non-malignant tissues (considering either primary lung tumors or lymph nodes)37, 46, 59 and also in Adk and Sqc60,61,62. However, literature data are really heterogeneous in this setting and, despite promising results, it isn’t possible to suggest for use a reproducible feature or a combination of features able to characterize definitely malignant tissues or lung cancer subtypes.
Again, different metrics, matrices, and methods (e.g., tumor segmentation, survival endpoints) have been reported in the evaluation of NSCLC prognosis based on PET features. Commonly, the term “heterogeneous” is used with different meanings. Concerning texture analysis, “heterogeneity” may result from one or more PET features positively or negatively related to treatment and/or outcome. Therefore clinical texture papers often use the term “heterogeneity” to summarize specific tumor characteristics expressed by PET features. Among these, “entropyHist” and “entropyGLCM” are most frequently reported to have an independent prognostic role, able to predict treatment outcome and/or survival in NSCLC patients13, 29, 35, 48, 78, 79, 83.
It is important for textural feature values to be directly comparable, both between and within patients, in order to derive meaningful conclusions from radiomic analysis and allow their use in clinical routine. However, the lack of a standardized method to calculate textural features prevents comparison between literature data and meta-analysis. Additionally, crucial information about texture extraction is not always available within the published articles. In order to try to simplify the complexity of texture analysis and to facilitate comparison among different series, some mandatory information concerning specific methodological aspects should be reported. Specifically, in the drafting of a paper on PET texture analysis, the following should be indicated in the Methods section (Table 4). Specifically, in the drafting of a paper on PET texture analysis, the following should be indicated in the Methods section: (a) the scanner, (b) the method (e.g., respiratory motion, dynamic) and parameters used to (c) acquire and (d) reconstruct images, (e) the type of images used to extract features (i.e., PET or both PET and CT), (f) the “target” of texture analysis (e.g., primary tumor, lymph nodes), (g) the application of PVC and/or a minimum lesion size/volume, (h) the method of segmentation (e.g., threshold uptake 40% of the SUVmax), (i) the discretization method (e.g., fixed number of bins), (j) the software, and (k) the feature(s) and matrix computation method. As the published results are very preliminary, a preferred method for reconstruction, discretization, or segmentation cannot be recommended. Obviously, consistent terminology in respect of features (a proposal is reported within the Supplementary material) and an appropriate statistical analysis are mandatory. Datasets of 10–15 patients per feature have been recommended to test the prognostic power of texture features. Moreover, use of the radiomics features insensitive to acquisition modes and reconstruction parameters is recommended. The correlation of conventional metrics (SUV, MTV, etc.) and texture features should be assessed to evaluate the potential complementary value of the measures. In addition, independent validation datasets are needed to confirm the results. Finally, there is a need for easy-to-use software tools for feature extraction (their main characteristics have been very recently summarized by Hatt et al.85) since those available are handled only by non-clinician experts, are time consuming, and are able to produce a lot of textures from different matrices, many of which are probably unnecessary since their biologic significance is encapsulated within others.
In conclusion, standardization is mandatory to prove the value of the information that can be derived from medical images, enabling non-invasive in vivo characterization of lung lesions and accurate risk stratification for the purpose of decision making regarding treatment strategy.
Ethical approval
This article does not contain any study with human participants or animals. The patient imaged in the Fig. 3, signed an inform consent to use his personal data including imaging, also for publication; however the figure is completely anonymized, preventing the possibility of discovering the identity of the individual.
References
Tixier, F. et al. Reproducibility of Tumor Uptake Heterogeneity Characterization Through Textural Feature Analysis in 18F-FDG PET. J. Nucl. Med. 53, 693–700 (2012).
Sauter, A. W., Schwenzer, N., Divine, M. R., Pichler, B. J. & Pfannenberg, C. Image-derived biomarkers and multimodal imaging strategies for lung cancer management. Eur. J. Nucl. Med. Mol. Imaging 42, 634–643 (2015).
Meignan, M., Itti, E., Gallamini, A. & Younes, A. FDG PET/CT imaging as a biomarker in lymphoma. Eur. J. Nucl. Med. Mol. Imaging 42, 623–633 (2015).
Differding, S., Hanin, F.-X. & Grégoire, V. PET imaging biomarkers in head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 42, 613–622 (2015).
Picchio, M. et al. Predictive value of pre-therapy (18)F-FDG PET/CT for the outcome of (18)F-FDG PET-guided radiotherapy in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 41, 21–31 (2014).
Guo, W. et al. Prediction of clinical phenotypes in invasive breast carcinomas from the integration of radiomics and genomics data. J. Med. imaging (Bellingham, Wash.) 2, 41007 (2015).
Wang, J. et al. Identifying Triple-Negative Breast Cancer Using Background Parenchymal Enhancement Heterogeneity on Dynamic Contrast-Enhanced MRI: A Pilot Radiomics Study. PLoS One 10, e0143308 (2015).
Yip, S. S. F. et al. Relationship between the Temporal Changes in Positron-Emission-Tomography-Imaging-Based Textural Features and Pathologic Response and Survival in Esophageal Cancer Patients. Front. Oncol. 6, 72 (2016).
Hyun, S. H. et al. Intratumoral heterogeneity of (18)F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging. doi:10.1007/s00259-016-3316-6 (2016).
Rahim, M. K. et al. Recent Trends in PET Image Interpretations Using Volumetric and Texture-based Quantification Methods in Nuclear Oncology. Nucl. Med. Mol. Imaging (2010). 48, 1–15 (2014).
O’Connor, J. P. B. et al. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin. Cancer Res. 21, 249–257 (2015).
Aerts, H. J. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5, 4006 (2014).
Win, T. et al. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin. Cancer Res. 19, 3591–3599 (2013).
Chicklore, S. et al. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur. J. Nucl. Med. Mol. Imaging 40, 133–140 (2013).
Buvat, I., Orlhac, F. & Soussan, M. Tumor Texture Analysis in PET: Where Do We Stand? J. Nucl. Med. 56, 1642–1644 (2015).
Zorzela, L. et al. PRISMA harms checklist : improving harms reporting in systematic reviews. BMJ 352, i157 (2016).
Al-Kadi, O. S. & Watson, D. Texture analysis of aggressive and nonaggressive lung tumor CE CT images. IEEE Trans. Biomed. Eng. 55, 1822–1830 (2008).
Ganeshan, B., Miles, K. A., Young, R. C. D. & Chatwin, C. R. Hepatic entropy and uniformity: additional parameters that can potentially increase the effectiveness of contrast enhancement during abdominal CT. Clin. Radiol. 62, 761–768 (2007).
Brown, R. a. & Frayne, R. A comparison of texture quantification techniques based on the Fourier and S transforms. Med. Phys. 35, 4998–5008 (2008).
Craciunescu, O. I., Das, S. K. & Clegg, S. T. Dynamic contrast-enhanced MRI and fractal characteristics of percolation clusters in two-dimensional tumor blood perfusion. J. Biomech. Eng. 121, 480–486 (1999).
Goh, V., Sanghera, B., Wellsted, D. M., Sundin, J. & Halligan, S. Assessment of the spatial pattern of colorectal tumour perfusion estimated at perfusion CT using two-dimensional fractal analysis. Eur. Radiol. 19, 1358–1365 (2009).
Dettori, L. & Semler, L. A comparison of wavelet, ridgelet, and curvelet-based texture classification algorithms in computed tomography. Comput. Biol. Med. 37, 486–498 (2007).
Sanghera, B. et al. Reproducibility of 2D and 3D Fractal Analysis Techniques for the Assessment of Spatial Heterogeneity of Regional Blood Flow in Rectal Cancer. http://dx.doi.org/10.1148/radiol.12111316 (2012).
Al-Kadi, O. S. Assessment of texture measures susceptibility to noise in conventional and contrast enhanced computed tomography lung tumour images. Comput. Med. Imaging Graph. 34, 494–503 (2010).
Tuceryan, M., Tuceryan, M., Jain, A. K. & Jain, A. K. The Handbook of Pattern Recognition and Computer Vision (2nd Edition), Texture Analysis. Pattern Recognit. 207–248, doi:10.1097/RCT.0b013e3181ec05e4 (1998).
Yip, S. S. F. & Aerts, H. J. W. L. Applications and limitations of radiomics. Phys. Med. Biol. 61, R150–R166 (2016).
El Naqa, I. et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 42, 1162–1171 (2009).
Drzymala, R. E. et al. Dose-volume histograms. Int. J. Radiat. Oncol. Biol. Phys. 21, 71–78 (1991).
Vaidya, M. et al. Combined PET/CT image characteristics for radiotherapy tumor response in lung cancer. Radiother. Oncol. 102, 239–245 (2012).
Nestle, U. et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J. Nucl. Med. 46, 1342–1348 (2005).
Van Velden, F. H. P. et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur. J. Nucl. Med. Mol. Imaging 38, 1636–1647 (2011).
Boellaard, R. et al. A novel cumulative SUV- volume histogram method for parameterizing heterogeneous tumour tracer uptake in oncology FDG PET studies. Eur. J. Nucl. Med. Mol. Imaging 37, S261 (2010).
Haralick, R., Shanmugam, K. & Dinstein, I. Texture Features for Image Classification. IEEE Trans Sys Man Cyb SMC. 3, 610–621 (1973).
Yan, R. et al. Detection of Myocardial Metabolic Abnormalities by 18F-FDG PET/CT and Corresponding Pathological Changes in Beagles with Local Heart Irradiation. Korean J. Radiol. 16, 919–928 (2015).
Tixier, F. et al. Visual Versus Quantitative Assessment of Intratumor 18F-FDG PET Uptake Heterogeneity: Prognostic Value in Non-Small Cell Lung Cancer. J. Nucl. Med. 55, 1235–1241 (2014).
Lovinfosse, P. et al. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur. J. Nucl. Med. Mol. Imaging, doi:10.1007/s00259-016-3314-8 (2016).
Miwa, K. et al. FDG uptake heterogeneity evaluated by fractal analysis improves the differential diagnosis of pulmonary nodules. Eur. J. Radiol. 83, 715–719 (2014).
Grigsby, P. W., Siegel, B. A., Dehdashti, F., Rader, J. & Zoberi, I. Posttherapy [18F] fluorodeoxyglucose positron emission tomography in carcinoma of the cervix: response and outcome. J. Clin. Oncol. 22, 2167–2171 (2004).
Greven, K. M. Positron-emission tomography for head and neck cancer. Semin. Radiat. Oncol. 14, 121–129 (2004).
Leijenaar, R. T. H. et al. Stability of FDG-PET Radiomics features: an integrated analysis of test-retest and inter-observer variability. Acta Oncol. 52, 1391–1397 (2013).
Velden, F. H. P. V et al. Repeatability of Radiomic Features in Non-Small-Cell Lung Cancer [18 F] FDG-PET/CT Studies : Impact of Reconstruction and Delineation. Mol Imaging Biol, doi:10.1007/s11307-016-0940-2 (2016).
Oliver, J. A. et al. Variability of Image Features Computed from Conventional and Respiratory-Gated PET/CT Images of Lung Cancer. Transl. Oncol. 8, 524–534 (2015).
Depeursinge, A., Foncubierta-Rodriguez, A., Van De Ville, D. & Müller, H. Three-dimensional solid texture analysis in biomedical imaging: Review and opportunities. Med. Image Anal. 18, 176–196 (2014).
Hatt, M. et al. Impact of Tumor Size and Tracer Uptake Heterogeneity in 18F-FDG PET and CT Non-Small Cell Lung Cancer Tumor Delineation. J. Nucl. Med. 52, 1690–1697 (2011).
Dong, X. et al. Intra-tumour (18) F-FDG uptake heterogeneity decreases the reliability on target volume definition with positron emission tomography/computed tomography imaging. J. Med. Imaging Radiat. Oncol. d, 338–345 (2015).
Orlhac, F. et al. Tumor Texture Analysis in 18F-FDG PET: Relationships Between Texture Parameters, Histogram Indices, Standardized Uptake Values, Metabolic Volumes, and Total Lesion Glycolysis. J. Nucl. Med. 55, 414–422 (2014).
Yan, J. et al. Impact of Image Reconstruction Settings on Texture Features in 18 F-FDG PET. J nucl med 56, 1667–1674 (2015).
Cheng, N. M., Dean Fang, Y. H., Tsan, D. L., Hsu, C. H. & Yen, T. C. Respiration-averaged CT for attenuation correction of PET Images - Impact on PET Texture Features in Non-Small Cell Lung Cancer Patients. PLoS One 11, 1–15 (2016).
Hofheinz, F. et al. An automatic method for accurate volume delineation of heterogeneous tumors in PET. Med. Phys. 40, 82503 (2013).
Cui, H., Wang, X. & Feng, D. Automated localization and segmentation of lung tumor from PET-CT thorax volumes based on image feature analysis. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, 5384–5387 doi:10.1109/EMBC.2012.6347211 (2012).
Cui, H., Wang, X., Zhou, J. & Eberl, S. Topology polymorphism graph for lung tumor segmentation in PET-CT images. Phys. Med. Biol. 4893, 4893 (2015).
Leijenaar, R. T. H. et al. The effect of SUV discretization in quantitative FDG-PET Radiomics: the need for standardized methodology in tumor texture analysis. Sci. Rep. 5, 11075 (2015).
Orlhac, F., Soussan, M., Chouahnia, K., Martinod, E. & Buvat, I. 18F-FDG PET-derived textural indices reflect tissue-specific uptake pattern in non-small cell lung cancer. PLoS One 10, 1–16 (2015).
Boellaard, R. et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur. J. Nucl. Med. Mol. Imaging 42, 328–354 (2014).
Tixier, F. et al. Comparison of tumor uptake heterogeneity characterization between static and parametric 18F-FDG PET images in Non-Small Cell Lung Cancer. J. Nucl. Med. 31 (2016).
Yip, S. et al. Comparison of texture features derived from static and respiratory-gated PET images in non-small cell lung cancer. PLoS One 9, e115510 (2014).
Gao, X. et al. The method and efficacy of support vector machine classifiers based on texture features and multi-resolution histogram from 18F-FDG PET-CT images for the evaluation of mediastinal lymph nodes in patients with lung cancer. Eur. J. Radiol. 84, 312–317 (2015).
Buvat, I., Orlhac, F. & Soussan, M. Tumor Texture Analysis in PET: Where Do We Stand? J. Nucl. Med. 56, 1642–1644 (2015).
Budiawan, H. et al. Heterogeneity Analysis of 18F-FDG Uptake in Differentiating Between Metastatic and Inflammatory Lymph Nodes in Adenocarcinoma of the Lung: Comparison with Other Parameters and its Application in a Clinical Setting. Nucl. Med. Mol. Imaging (2010). 47, 232–241 (2013).
Ha, S. et al. Autoclustering of Non-small Cell Lung Carcinoma Subtypes on 18F-FDG PET Using Texture Analysis: A Preliminary Result. Nucl. Med. Mol. Imaging (2010). 48, 278–286 (2014).
Kim, D.-H. et al. Prognostic Significance of Intratumoral Metabolic Heterogeneity on 18F-FDG PET/CT in Pathological N0 Non–Small Cell Lung Cancer. Clin. Nucl. Med. 40, 708–714 (2015).
van Gómez López, O. et al. Heterogeneity in [18 F] fluorodeoxyglucose positron emission tomography/computed tomography of non – small cell lung carcinoma and its relationship to metabolic parameters and pathologic staging. Mol. Imaging 13, 1–12 (2014).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Warburg, O. On respiratory impairment in cancer cells. Science 124, 269–270 (1956).
Mathupala, S. P., Ko, Y. H. & Pedersen, P. L. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the & quot; Warburg Effect & quot; and a pivotal target for effective therapy. Semin. Cancer Biol. 19, 17–24 (2009).
Frezza, C. & Gottlieb, E. Mitochondria in cancer: Not just innocent bystanders. Semin. Cancer Biol. 19, 4–11 (2009).
Robey, R. B. & Hay, N. Is Akt the & quot; Warburg kinase & quot; ?-Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 19, 25–31 (2009).
Dang, C. V. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle 9, 3884–3886 (2010).
Dang, C. V. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 70, 859–862 (2010).
Dang, C. V. Enigmatic MYC Conducts an Unfolding Systems Biology Symphony. Genes Cancer 1, 526–531 (2010).
Nair, V. S. et al. Prognostic PET 18F-FDG uptake imaging features are associated with major oncogenomic alterations in patients with resected non-small cell lung cancer. Cancer Res. 72, 3725–3734 (2012).
Chalkidou, A., O’Doherty, M. J. & Marsden, P. K. False discovery rates in PET and CT studies with texture features: A systematic review. PLoS One 10, 1–18 (2015).
Apostolova, I. et al. Quantitative assessment of the asphericity of pretherapeutic FDG uptake as an independent predictor of outcome in NSCLC. BMC Cancer 14, 896 (2014).
Hatt, M. et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J. Nucl. Med. 56, 38–44 (2015).
Kang, S.-R. et al. Intratumoral Metabolic Heterogeneity for Prediction of Disease Progression After Concurrent Chemoradiotherapy in Patients with Inoperable Stage III Non-Small-Cell Lung Cancer. Nucl. Med. Mol. Imaging (2010). 48, 16–25 (2014).
Ohri, N. et al. Pretreatment 18FDG-PET Textural Features in Locally Advanced Non-Small Cell Lung Cancer: Secondary Analysis of ACRIN 6668/RTOG 0235. J. Nucl. Med. 1–30, doi:10.2967/jnumed.115.166934 (2016).
Cook, G. J. R. et al. Are Pretreatment 18F-FDG PET Tumor Textural Features in Non–Small Cell Lung Cancer Associated with Response and Survival After Chemoradiotherapy? J. Nucl. Med. 54, 19–26 (2013).
Cook, G. J. R. et al. Non-Small Cell Lung Cancer Treated with Erlotinib: Heterogeneity of (18)F-FDG Uptake at PET-Association with Treatment Response and Prognosis. Radiology 276, 883–893 (2015).
Pyka, T. et al. Textural features in pre-treatment [F18]-FDG-PET/CT are correlated with risk of local recurrence and disease-specific survival in early stage NSCLC patients receiving primary stereotactic radiation therapy. Radiat. Oncol. 10, 100 (2015).
Carvalho, S. et al. Prognostic value of metabolic metrics extracted from baseline PET images in NSCLC in non small cell lung cancer. Acta Oncol 52, 1398–1404 (2013).
Fried, D. V. et al. Stage III Non-Small Cell Lung Cancer: Prognostic Value of FDG PET Quantitative Imaging Features Combined with Clinical Prognostic Factors. Radiology 278, 214–222 (2016).
Fried, D. V. et al. Potential Use of 18F-fluorodeoxyglucose Positron Emission Tomography-Based Quantitative Imaging Features for Guiding Dose Escalation in Stage III Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 94, 368–376 (2016).
Desseroit, M.-C. et al. Development of a nomogram combining clinical staging with (18) F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur. J. Nucl. Med. Mol. Imaging, doi:10.1007/s00259-016-3325-5 (2016).
Wu, J. & Rubin, D. L. Early-Stage Non – Small Cell Lung Cancer : Quantitative Imaging Characteristics of 18 F Fluorodeoxyglucose PET/CT Allow. Radiology (2016).
Hatt, M. et al. Characterization of PET/CT images using texture analysis: the past, the present … any future? Eur. J. Nucl. Med. Mol. Imaging, doi:10.1007/s00259-016-3427-0 (2016).
Author information
Authors and Affiliations
Contributions
The manuscript has been seen and approved by all authors, whose individual contributions were as follows: K.M. and S.M. conceptualized the paper, K.M. and L.A. contributed to data selection; K.M. and S.M., wrote the paper; K.M. and S.M. provided figures and tables; L.C. revised and checked the nomenclature and made uniform the formulas of the supplemental material; C.A. supervised the project and paper writing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sollini, M., Cozzi, L., Antunovic, L. et al. PET Radiomics in NSCLC: state of the art and a proposal for harmonization of methodology. Sci Rep 7, 358 (2017). https://doi.org/10.1038/s41598-017-00426-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00426-y
This article is cited by
-
Identification of CT radiomic features robust to acquisition and segmentation variations for improved prediction of radiotherapy-treated lung cancer patient recurrence
Scientific Reports (2024)
-
Long-axial field-of-view PET/CT: perspectives and review of a revolutionary development in nuclear medicine based on clinical experience in over 7000 patients
Cancer Imaging (2023)
-
Deep learning model integrating positron emission tomography and clinical data for prognosis prediction in non-small cell lung cancer patients
BMC Bioinformatics (2023)
-
Radiomic analysis will add differential diagnostic value of benign and malignant pulmonary nodules: a hybrid imaging study based on [18F]FDG and [18F]FLT PET/CT
Insights into Imaging (2023)
-
Shape and texture analyses based on conventional MRI for the preoperative prediction of the aggressiveness of pituitary adenomas
European Radiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.