Abstract
Obesity has been well studied in relation to breast cancer survival. However, the associations of post-diagnosis obesity and late outcomes (≥5 years after diagnosis) have been much less studied. A total of 4062 5-year disease-free patients were recruited from the Shanghai Breast Cancer Survival Study, a longitudinal study of patients diagnosed during 2002-2006. Cox proportional hazard model with restricted cubic spline were used to evaluate the potential non-linear associations of post-diagnosis body mass index (BMI) and waist-to-hip ratio (WHR) with late all-cause mortality and late recurrence. While no significant association was observed for post-diagnosis BMI or WHR with late recurrence; a U-shaped association was observed for the two measures with late all-cause death. Women with BMI of 25.0 kg/m2 or WHR of 0.83 were at the lowest risk of late all-cause mortality, whereas those with BMI beyond the range of 22.1–28.7 kg/m2 or WHR beyond the range of 0.81–0.86 had a higher risk. ER, stage or menopausal status did not modify the effect of post-diagnosis BMI or WHR on the outcomes. In conclusion, post-diagnosis BMI and WHR, as indicators of overall and central obesity respectively, were associated with late all-cause mortality in U-shaped pattern among long-term breast cancer survivors.
Similar content being viewed by others
Introduction
Body mass index (BMI) has been consistently associated with both all-cause mortality and recurrence in a U-shaped or J-shaped pattern1,2,3,4,5,6. By contrast, waist-to-hip ratio (WHR), an index of central obesity, was much less studied, and the results were mixed. Most studies1, 2, 7,8,9, not all3, 10 suggested that WHR was positively associated with breast cancer survival. With the increasing 5-year survival rate in breast cancer patients around the world, including China11, more concern has been aroused on the role of obesity in late outcomes of breast cancer. Breast cancer patients may have a considerable residual risk of recurrence in later years, especially in estrogen receptor (ER)-positive patients who were treated with adjuvant endocrine therapy12, 13.
Several studies had divided events into those occurred within and after 5 or 10 years after diagnosis to identify prognostic factors of late outcomes, such as obesity, tumor size, nodal status, tumor grade and recurrence score14,15,16,17,18,19. Among them, only two studies investigated the associations between obesity and late outcomes14, 19. In the two studies, obesity (BMI ≥ 30 kg/m2) or severe obesity (BMI ≥ 35 kg/m2) post-diagnosis or at diagnosis were found in relation to higher hazard of all-cause death, breast cancer death and recurrence 5–10 years after diagnosis. However, no studies have ever evaluated the association between WHR and late outcomes.
In our previous report based on the Shanghai Breast Cancer Survival Study (SBCSS), we found that obesity (BMI > 30 kg/m2) measured at 6 months after diagnosis was inversely related to breast cancer prognosis after a median follow-up time of 46 months (i.e. early outcomes)3, but did not observe a significant association for WHR. In this study, we further followed up the cancer cases to explore the relationship of both indicators with late all-cause mortality and late recurrence, i.e. events occurring 5 years after diagnosis. In addition to using a traditional analysis method, which categorizes BMI and WHR according to World Health Organization standard20 or quartile distributions, we applied Cox proportional hazard model with restricted cubic spline (RCS) to reveal the potential non-linearity associations of BMI and WHR with late outcomes.
Results
Demographic, clinical and lifestyle factors in 5-year disease-free patients
Among 4062 5-year disease-free patients, 326 deaths and 264 recurrences occurred ≥ 5 years after diagnosis. The median follow-up time for late all-cause mortality and late recurrence were 10.54 years (5.02–12.78) and 8.40 (5.01–11.03), respectively.
Table 1 shows demographic characteristics, clinical predictors and lifestyle factors of 5-year disease-free patients assessed at 6 months after diagnosis, as well as BMI, WHR and menopausal status measured at 60 months’ post-diagnosis. In the patients, 73.4% were at early stage (I-IIA) of breast cancer, more than 65.5% were ER-positive, 5.0% were general obese and 39.3% were central obese at baseline. BMI and WHR at 6 months’ post-diagnosis were significantly correlated, with a Pearson correlation coefficient of 0.47 (P < 0.001). Pearson correlation coefficient demonstrated that WHR was more correlated with waist circumference (r = 0.75) than hip circumference (r = 0.25).
Associations of BMI and WHR with late outcomes
Table 2 presents the associations of BMI and WHR as categorical variables with late outcomes of breast cancer. After adjusting for demographic, clinical and lifestyle factors, no significant associations were observed for BMI and WHR with later recurrence; but U-shaped associations were observed for BMI and WHR with late all-cause mortality.
Further analysis in Cox models with RCS found that both BMI and WHR were significantly related to late all-cause mortality (P = 0.01 and <0.001 respectively) (Table 3). The associations were in nonlinear U-shaped pattern (P = 0.003 and <0.001 respectively), and patients with BMI beyond (below or above) the range of 22.1–28.7 kg/m2 having a higher risk of death (Fig. 1a and b). Compared with patients with BMI of 25.0 kg/m2 (reference BMI with lowest hazard), those with BMI at 1st percentile (near 17.0 kg/m2) had 86% increased risk (95% CI: 1.17–2.98), and those with BMI at 99th percentile (near 34.0 kg/m2) had 59% increased risk (95% CI: 1.08–2.34). Further adjustment for WHR did not substantially change the pattern. A similar but more pronounced association pattern was observed for WHR with late all-cause mortality. However, as shown in Table 3, no significant association was observed for BMI and WHR with late recurrence (P = 0.240 and 0.054, respectively).
Associations of BMI and WHR with late outcomes by ER status, TNM stage and menopausal status
In further stratified analysis, the relationship between BMI and late all-cause mortality did not vary greatly by ER status, TNM stage and menopausal status (P for interaction = 0.327, 0.610 and 0.569 respectively), although the U-shaped associations appeared more pronounced in ER-negative and postmenopausal patients, and the association of lower BMI with higher hazard was more prominent in patients with stage I breast cancer (Table 4). In addition, ER status itself was a significant prognostic factor, with ER-positive status related with 36% (95% CI: 1.06–1.74) higher risk of late all-cause mortality.
As shown in Table 5, a borderline significant modifying effect was observed for ER status in the association between WHR and late all-cause mortality (P for interaction = 0.087). Among ER-negative patients, comparing with those having the lowest risk (WHR of 0.85), women with WHR of 0.70 had 4-fold risk of late mortality, and women with WHR of 1.0 had a doubled risk. In ER-positive patients, on the other hand, comparing with those having the lowest risk (WHR of 0.83), women with WHR of 0.70 and 1.0 had 44% increased risk and nearly 3-fold risk of late mortality, respectively.
Due to small sample size (436) and number of events (11) in premenopausal women, we could not evaluate the potential modifying effect of menopausal status on the U-shape associations of WHR and BMI with later all-cause mortality. No significant modifying effect was observed for age at diagnosis (<65/ ≥ 65 years old), physical activity (Yes/No) and comorbidity (Yes/No), either.
Discussion
In this cohort study of 4062 5-year disease-free patients, we found that BMI and WHR were associated with late all-cause mortality in a U-shaped pattern. The lowest hazard was observed in patients with BMI of 25.0 kg/m2 or in patients with WHR of 0.84, and a higher risk was found in patients with BMI beyond the range of 22.1–28.7 kg/m2 or in those with WHR beyond the range of 0.81–0.86. Neither BMI nor WHR was related to late recurrence. To our knowledge, this study is the first one to evaluate the effect of WHR on late outcomes in long-term breast cancer survivors.
Our finding of the U-shaped association of BMI with late all-cause mortality was consistent with some of the previous studies14, 19, in which one study in Denmark found that positive association of obesity (BMI ≥ 30 kg/m2) at diagnosis with the risk of distant metastasis or breast cancer death were stronger beyond than within 5 years after diagnosis19. In a pooled analysis on ER-positive breast cancer14, U-shaped and J-shaped associations were detected for 4.6 years’ post-diagnosis BMI with late all-cause mortality and late recurrence, respectively. Our findings, as well as the positive association of obesity with early outcome of breast cancer previously reported in this population3, provide strong support for the predictive role of obesity in long-term survival of breast cancer patients.
Obesity has been consistently found a negative prognostic factor in breast cancer patients, but the effect of underweight remains unclear. In this study, we found that women with BMI <22.1 kg/m2, including those underweighted and patients with normal-low BMI, had elevated risk of death. As all patients in our study were long-term survivors who went through long course of disease, the inhibited immune system and cytokine reactions caused by chronic malnutrition21, and weight loss resulted from illness might explain the result. Unintentional weight loss caused by cachexia could be the reason for the association between lower BMI and increased risk of death. However, cachexia was not documented in our study and we were not able to evaluate its role in the mechanism. Moreover, lower BMI could be due to preexisting comorbidities that had already placed these women at greater risk of poor outcomes. In our study, we adjusted for comorbidity as Charlson comorbidity index ≥ 1 vs. 0, where index ≥ 1 meant the patient had at least one comorbid condition among the scoring range of Charlson comorbidity index, and the association estimates did not differ by the two strata. Further analysis using original continuous scores of Charlson comorbidity did not change the lower BMI/WHR-mortality associations. However, residual confounding by other unmeasured comorbidities outside the scoring range of Charlson comorbidity index could be possible. It’s noteworthy that the negative prognostic effect of underweight was more likely to be observed in Asian populations4,5,6, 22. In 24,698 Korean breast cancer patients, underweighted (<18.5 kg/m2) patients were observed to have a significantly poorer overall survival and shorter time to distant and local recurrence compared with patients with normal weight (18.5–24.9 kg/m2)5. The study conducted in 20,090 Japanese cases also observed a significant underweight-mortality association in all and postmenopausal breast cancer patients22.
Comparing with BMI, WHR, as an approximation of visceral adipose tissue, is a better measurement for central obesity and elderly people23. However, the prognostic effect of WHR in breast cancer was much less studied. While several long-term follow-up studies (more than 10 years) reported an increased risk of all-cause death in the highest vs. the lowest quartile groups of WHR1, 2, 7,8,9, 24, the other two studies with follow-up time around 5 years did not find a significant association3, 10. In this study, we observed, for the first time, a U-shaped association between WHR and late all-cause mortality, in which a higher or a lower WHR was related with a higher risk of death. In the former report of our cohort, HR in each quartile of WHR also presented a U-shaped pattern, although the association was not significant3. This indicated that the lack of significance might be attributable to using traditional analysis method by categorizing predictor, which reduced statistical power.
The mechanisms underlying the U-shaped pattern are unclear. 82.7% of the women in our study were post-menopausal at about 5 years after their diagnosis. They tend to lose lean body mass and have a shift of body fat from peripheral to central sites with an accompanying increase in WHR at the same level of BMI23. Higher WHR representing higher VAT is related to insulin resistance, hyperinsulinemia, adipose-derived hormones and chronic inflammation, which are thought to play an important role in carcinogenesis25, 26. Insulin further stimulates the production of estrogen and the expression of ER-α in breast cancer cells27. As to the patients with low WHR, it is speculated that inhibited immune system caused by undernutrition, which justify the association of lower BMI with higher mortality might also be applicable to the link between low WHR and higher mortality. Due to limited studies on WHR, these associations need to be verified in future studies, especially in Asians.
Results of studies evaluating the effect of BMI according to hormone receptor status were inconsistent. It has been indicated that the effect of general obesity on breast cancer prognosis may be stronger in women with ER-positive tumors than women with ER-negative tumors28, 29. But, a meta-analysis of 21 studies found no evidence that the relation of obesity to breast cancer outcomes varies by hormone receptor status30. Moreover, results from the randomized SUCCESS A trial demonstrated that the adverse effect of severe obesity was only found in triple negative breast cancer subgroup rather than luminal or HER2-positive subtypes31. In our study, the association appeared to be more apparent in ER-negative patients in our study, although the interaction was not significant. It might be speculated that the association in ER-positive patients could have been masked by the benefit of endocrine therapies such as tamoxifen or aromatase inhibitor.
Positive relation of WHR to breast cancer mortality was restricted to ER-positive postmenopausal women in a study of Vancouver, Canada8, whereas no modifying effect was found in other two studies1, 2. Our study suggested a significant association of WHR with late all-cause mortality among both women with ER-negative and ER-positive breast cancer. The lower WHR-mortality relation was more obvious in ER-negative patients; while the higher WHR-mortality relation was more apparent in ER-positive patients, where the estrogen mediated mechanism could justify this association8.
Recent studies observed varying associations between BMI and survival of colorectal cancer32, ovarian cancer33 and many other types of cancer34 across TNM stages. As to breast cancer, an Italian study observed an unfavorable effect of high BMI only in women with stage I-II breast cancer1. However, no formal interaction test was performed and the sample size was small in advanced-stage patients. Another study found that the association was consistent across strata of cancer stages35. In our study, although the interaction test was not significant, we observed a significant association between BMI and late all-cause mortality in stage I patients, but not among those with stage III-IV cancer. However, the null association in the stage III-IV patients could be due to small sample size and rare events in this stratum. Further study involving larger number of advanced breast cancers is warranted. In addition, BMI-mortality association was more pronounced in patients with lower BMI than in patients with higher BMI in stage I patients. This could be due to the fact that patients with higher BMI tend to be diagnosed with later stage35. Interestingly, in line with previous studies conducted in US populations36, 37, we find that the effect of ER status in breast cancer survival was also time-dependent in Chinese patients. ER-positive status was a protective factor for early outcomes38, but a risk factor for late outcomes (≥5 years) which may provide reference for the treatment of breast cancer.
The main strengths of this study included the large sample size, more than 10 years’ follow-up time for all-cause death, detailed information on post-diagnostic lifestyle, clinical factors and accurate anthropometric measurements taken at 6 and 60 months after diagnosis, and use of RCS in data analysis. The RCS, by using all information, increasing statistical power, and testing non-linear relationship, has enabled us to evaluate the risk for each value of BMI and WHR and to identify the lowest risk point of BMI and WHR. However, several limitations should be mentioned. First, the average follow-up time for recurrence was only 8.4 years, relatively shorter than that for all-cause mortality, which may had led to small power to evaluate the association of obesity with risk of recurrence. Moreover, we just treated BMI and WHR as time-dependent variables, but not for other variables including ER status, which may also have changed during the 10-year follow-up time. This may introduce bias to our results.
In summary, our findings of the U-shaped associations between BMI, WHR and late all-cause mortality provide strong evidence on the long-term effect of obesity on breast cancer survival, and indicate the benefits of keeping moderate body size for breast cancer patients. Further studies are warranted to evaluate the potential modifying effect of ER and menopausal status in the associations.
Methods
Study population
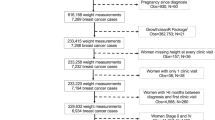
Patients included in this study were from the SBCSS, a longitudinal, population-based study of women aged 20 to 75 years old who were diagnosed with primary breast cancer between March 2002 and April 2006. Details of the study design of the SBCSS have been described previously39. In brief, all patients were permanent residents of Shanghai, China, and from the Shanghai Cancer Registry. Among 6299 patients contacted, 5042 women provided written, informed consent and were recruited into the study approximately 6 months after diagnosis. Reasons for non-participation included: refusal (n = 757, 12.0%), absence during study enrollment (n = 258, 4.1%), unable to contact (n = 83, 1.3%), and other miscellaneous reasons such as health or communication problems (n = 159, 2.5%). 156 patients diagnosed with breast cancer in situ were further excluded. Of 4886 subjects, 4062 were 5-year disease-free patients without death/recurrence/loss to follow-up prior to 5 years after diagnosis and were included in the analysis.
Ethics
This study was approved by the institutional review board and ethic committee of Vanderbilt University and Shanghai Municipal Center for Disease Control and Prevention. The study was carried out in accordance with the relevant guidelines and regulations. All participants in this research provided their written consents.
Data collection
In-person interviews were conducted approximately 6, 18, 36, and 60 months after diagnosis using questionnaires, with follow-up rates of 91%, 84%, and 77%, respectively, for the 18-, 36-, and 60-month post-diagnosis interview. Information on demographics, cancer diagnosis and treatment, menstrual and reproductive factors, selected life style factors (soy protein intake, cigarette and alcohol use, physical activity, etc.), comorbidity, use of complementary and alternative medicine and quality of life were collected at baseline survey. Medical records were reviewed to collect clinical information on date of diagnosis, TNM stage, ER status, progesterone receptor status, surgery, radiotherapy, chemotherapy, immunotherapy and hormonal therapy. A Charlson comorbidity index covering 19 categories of comorbid conditions was created for each woman based on a validated comorbidity scoring system, with index 0 and ≥1 representing not having and having at least one comorbidity within the scoring categories, respectively40. Menopausal status was defined as cessation of menstruation for at least 12 months, excluding situation caused by pregnancy or breast-feeding and hormone-induced menopause.
Anthropometric measurements (height, weight, waist and hip circumference) were taken twice at 6 and 60 months after diagnosis by trained interviewers according to a standard protocol. Self-reported weight at 18 and 36 months after diagnosis were not used in this analysis. Detailed measurements method was described in our previous report3. Briefly, weight was measured to the nearest 0.1 kg using a digital weight scale that was calibrated every 6 months. Standing height and circumferences were measured to the nearest 0.1 cm. Waist circumference was measured at 2.5 cm above the umbilicus and hip circumference at the level of maximum width of the buttocks with the subject in a standing position. BMI (weight in kilograms divided by the square of height in meters) and WHR (waist circumference divided by hip circumference) were then calculated based on the measurements. All 4062 patients in this study took the first measurements (6 months’ post-diagnosis), and 3292 and 3304 patients were measured with BMI and WHR, respectively, at 60 months’ post-diagnosis.
Survival status of each participant was collected by data linkage with the Shanghai Vital Statistics database. The most recent linkage was conducted on December 31, 2014 for all-cause mortality and December 31, 2012 for recurrence.
Statistical analysis
Outcomes for this study were late (≥5 years) all-cause death and late disease-free survival with an event defined as recurrence, metastasis, or breast cancer specific death, whichever occurred first, and referred to as late recurrence for convenience. All the analyses were based on 5-year disease-free survivors, who survived more than 5 years after their cancer diagnosis and without recurrence or metastasis41. Survival time was calculated as the period from the date of diagnosis to the date of death (or recurrence for the recurrence analysis) or date of last contact (i.e., date of last follow-up survey or last registry linkage, whichever was most recent).
Cox proportional hazard model was used to estimate the associations of BMI, WHR with late outcomes. Log-log survival plot was applied to evaluate proportional hazard assumption for these two variables. At first, BMI or WHR were put into the model as categorical variables. They were categorized according to quartile distribution instead of World Health Organization classification20 in that sample size of underweight group (BMI <18.5 kg/m2) was too small to give stable estimation. And then, BMI or WHR were treated as continuous variables in the model, and restricted cubic spline function was utilized to examine non-linearity relationship between predictors and outcomes and visually demonstrate the relationship; knots were placed at the 5th, 50th, 95th percentiles42, 43, values of BMI and WHR with the lowest hazard were taken as reference to estimate hazard ratios (HR) and 95% confidence intervals (CI) for any other values. Two statistical tests were conducted during this procedure, one test was for the null hypothesis that the regression coefficients of both linear and nonlinear terms of the factor were equal to zero, and the result was presented as “P for overall association”; another test was for the regression coefficient of nonlinear term, i.e. spline variable, and “P for non-linearity” <0.05 indicated a non-linear association. BMI or WHR were included as time-dependent variables by counting process method to capture changes during the follow-up process. Both 6 and 60 months’ post-diagnosis measurements were used if available; otherwise only 6 months’ post-diagnosis measurements were used.
Confounders adjusted in the Cox model included age at diagnosis (continuous), soy protein intake (continuous), ER status (positive/negative/unknown), TNM stage (I/IIA/IIB/III-IV/unknown), mastectomy (yes/no), chemotherapy (yes/no), radiotherapy (yes/no), comorbidity (0/ ≥ 1), physical activity (yes/no), education (<high school/high school/ > high school) and menopausal status (premenopausal/postmenopausal/unknown). Binary variables entered the model directly and polytomous variables were treated as dummy variables. Soy protein intake and physical activity was assessed at 6 months’ post-diagnosis, and menopausal status was at 60 months’ post-diagnosis as many patients changed their status during the follow-up. Smoking and alcohol drinking rates were very low in our study population, with only 2.2%/3.1% of them were former smokers/drinkers and 0.5%/0.3% of them were current smokers/drinkers at baseline. Therefore, we were not able to adjust these two variables. Potential modifying effects of ER status, TNM stage, menopausal status, age at diagnosis (<65/≥65 years old), physical activity (Yes/No) and comorbidity (Yes/No) were tested using a multiplicative scale. Multiplicative interaction term was examined through likelihood ratio test, which compared the model including only main effects (reduced model) and the model including both main effects and interactive terms (full model). Further stratified analyses were conducted by ER status and menopausal status. All analyses and graphs were performed using SAS (version 9.4; SAS Institute, Cary, NC). RCS was completed by SAS macro %RCS44. Tests of statistical significance were two-sided, and P < 0.05 was considered as significant.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Dal Maso, L. et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. INT J CANCER. 123, 2188–2194 (2008).
Kwan, M. L. et al. Obesity and mortality after breast cancer by race/ethnicity: The California Breast Cancer Survivorship Consortium. AM J EPIDEMIOL. 179, 95–111 (2014).
Chen, X. et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 122, 823–833 (2010).
Kwan, M. L. et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 132, 729–739 (2012).
Moon, H. G., Han, W. & Noh, D. Y. Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J CLIN ONCOL. 27, 5899–5905 (2009).
Kawai, M. et al. Body mass index and survival after breast cancer diagnosis in Japanese women. BMC CANCER. 12, 149 (2012).
Abrahamson, P. E. et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 15, 1871–1877 (2006).
Borugian, M. J. et al. Waist-to-hip ratio and breast cancer mortality. AM J EPIDEMIOL. 158, 963–968 (2003).
George, S. M. et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res Treat. 146, 647–655 (2014).
Tao, M. H., Shu, X. O., Ruan, Z. X., Gao, Y. T. & Zheng, W. Association of overweight with breast cancer survival. AM J EPIDEMIOL. 163, 101–107 (2006).
Allemani, C. et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). LANCET. 385, 977–1010 (2015).
Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. LANCET. 365, 1687–1717 (2005).
Davies, C. et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. LANCET. 378, 771–784 (2011).
Nechuta, S. et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. INT J CANCER. 138, 2088–2097 (2016).
Kennecke, H. et al. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. ANN ONCOL. 18, 45–51 (2006).
Sestak, I. et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 105, 1504–1511 (2013).
Brewster, A. M. et al. Residual Risk of Breast Cancer Recurrence 5 Years After Adjuvant Therapy. J Natl Cancer Inst. 100, 1179–1183 (2008).
Sgroi, D. C. et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. LANCET ONCOL. 14, 1067–1076 (2013).
Ewertz, M. et al. Effect of obesity on prognosis after early-stage breast cancer. J CLIN ONCOL. 29, 25–31 (2011).
World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. WHO: Geneva: Switzerland, 1998.
Cunningham-Rundles, S., McNeeley, D. F. & Moon, A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 115, 1119–1128, 1129 (2005).
Kawai, M. et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database-Breast Cancer Registry. Cancer Med. 5, 1328–1340 (2016).
Zhang, X. et al. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 167, 886–892 (2007).
Sun, X. et al. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control. 26, 1803–1811 (2015).
Inoue, M. & Tsugane, S. Insulin resistance and cancer: epidemiological evidence. Endocr Relat Cancer. 19, F1–F8 (2012).
Duggan, C. et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J CLIN ONCOL. 29, 32–39 (2011).
Lorincz, A. M. & Sukumar, S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 13, 279–292 (2006).
Maehle, B. O., Tretli, S. & Thorsen, T. The associations of obesity, lymph node status and prognosis in breast cancer patients: dependence on estrogen and progesterone receptor status. APMIS. 112, 349–357 (2004).
Maehle, B. O. & Tretli, S. Pre-morbid body-mass-index in breast cancer: reversed effect on survival in hormone receptor negative patients. Breast Cancer Res Treat. 41, 123–130 (1996).
Niraula, S., Ocana, A., Ennis, M. & Goodwin, P. J. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 134, 769–781 (2012).
Widschwendter, P. et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. BREAST CANCER RES. 17, 129 (2015).
Kocarnik, J. M. et al. Relationship of prediagnostic body mass index with survival after colorectal cancer: Stage-specific associations. INT J CANCER. 139, 1065–1072 (2016).
Bandera, E. V. et al. Impact of body mass index on ovarian cancer survival varies by stage. Br J Cancer. doi:10.1038/bjc.2017.162 (2017).
Greenlee, H., Unger, J. M., LeBlanc, M., Ramsey, S. & Hershman, D. L. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol Biomarkers Prev. 26, 21–29 (2017).
Whiteman, M. K. et al. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 14, 2009–2014 (2005).
Natarajan, L. et al. Time-Varying Effects of Prognostic Factors Associated With Disease-Free Survival in Breast Cancer. AM J EPIDEMIOL. 169, 1463–1470 (2009).
Jatoi, I., Anderson, W. F., Jeong, J. H. & Redmond, C. K. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J CLIN ONCOL. 29, 2301–2304 (2011).
Su, Y. et al. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC CANCER. 11, 292 (2011).
Shu, X. O. et al. Soy food intake and breast cancer survival. JAMA. 302, 2437–2443 (2009).
Grunau, G. L., Sheps, S., Goldner, E. M. & Ratner, P. A. Specific comorbidity risk adjustment was a better predictor of 5-year acute myocardial infarction mortality than general methods. J CLIN EPIDEMIOL. 59, 274–280 (2006).
Bellera, C. A. et al. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC MED RES METHODOL. 10, 20 (2010).
Desquilbet, L. & Mariotti, F., Dose-response analyses using restricted cubic spline functions in public health research. STAT MED (2010).
Durrleman, S. & Simon, R. Flexible regression models with cubic splines. STAT MED. 8, 551–561 (1989).
Heinzl, H. & Kaider, A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 54, 201–208 (1997).
Acknowledgements
The study was supported by a grant from National Natural Science Foundation of China (Grant No. 81402734 to P.P. Bao), and grants from the Department of Defense Breast Cancer Research Program (DAMD 17-02-1-0607 to X.-O. Shu) and the National Cancer Institute (R01 CA118229 to X.-O. Shu), and a grant from the National Natural Science Foundation of China (Grant No. 11371100 to G.Y. Qin).
Author information
Authors and Affiliations
Contributions
All of the authors met the ICMJE recommendations for authorship. Y.Z. and X.-O.S. contributed to the study design; H.C. collected the data; M.L.Z. conducted data analysis and the writing of the manuscript; P.P.B. contributed to the interpretation of results; G.Y.Q. and W.H.X. revised the manuscript. All of the authors take responsibility for the integrity and accuracy of the study.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Cai, H., Bao, P. et al. Body mass index, waist-to-hip ratio and late outcomes: a report from the Shanghai Breast Cancer Survival Study. Sci Rep 7, 6996 (2017). https://doi.org/10.1038/s41598-017-07320-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07320-7
This article is cited by
-
Associations of adiposity and weight change with recurrence and survival in breast cancer patients: a systematic review and meta-analysis
Breast Cancer (2022)
-
Adiposity and cancer survival: a systematic review and meta-analysis
Cancer Causes & Control (2022)
-
Prognostic significance of abdominal obesity and its post-diagnosis change in a Chinese breast cancer cohort
Breast Cancer Research and Treatment (2022)
-
Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors
BMC Cancer (2021)
-
Clinical verification of body mass index and tumor immune response in patients with breast cancer receiving preoperative chemotherapy
BMC Cancer (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.